The equation for complete oxidation of glucose in anaerobic respiration is glucose > (1) molecular formulas (2) structural formulas (3) total number of atoms per molecule (4) total number of bonds per molecule:
Which Word Equation Represents A Neutralization Reaction. The ionic equation omits ions which act as spectators so we can split the reactants and the products into ions to see which are the spectators. Let�s consider a few neutralization reactions and how we write the equations. We can write the overall equation for the reaction first. This uses the scientific names for the acid and alkali placed on the reactant side of the equation.
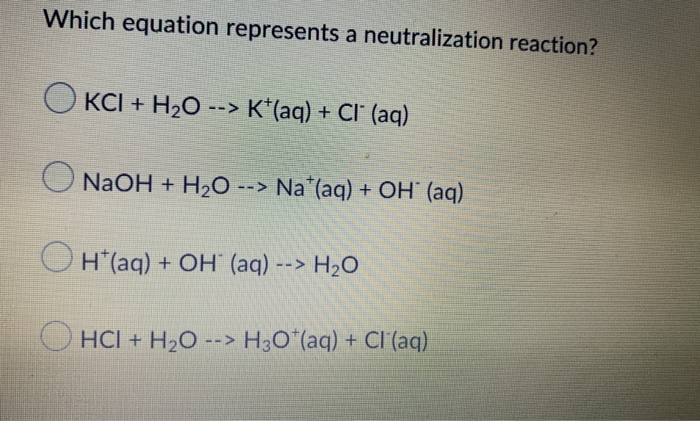 Solved Which Equation Represents A Neutralization Reaction? | Chegg.com From chegg.com
Solved Which Equation Represents A Neutralization Reaction? | Chegg.com From chegg.com
Related Post Solved Which Equation Represents A Neutralization Reaction? | Chegg.com :
This is the reaction that always occurs when an acid + alkali salt + water. Discusses chemical reactions that occur when acids and bases interact. This uses the scientific names for the acid and alkali placed on the reactant side of the equation. Let’s see how a neutralization reaction produces both water and a salt, using as an example the reaction between solutions of hydrochloric acid and sodium hydroxide.
Let’s see how a neutralization reaction produces both water and a salt, using as an example the reaction between solutions of hydrochloric acid and sodium hydroxide.
This uses the scientific names for the acid and alkali placed on the reactant side of the equation. Both have 4 carbons but there structures are different: The acid neutralizes the base, and hence, this reaction is called a neutralization reaction. The word equation for neutralization is acid + base = salt + water. When these two chemicals are mixed together, they create a solution of water, or h2o, and potassium sulfate, a salt. Which word equation represents a neutralization reaction?
 Source: yumpu.com
Source: yumpu.com
A) salt + acid ® base + water b) base + acid ® salt + water c) salt + water ® acid + base d) base + salt ® water + acid 1 (1) base + acid => salt + water (2) base +salt => water + acid (3) salt + acid =>base + water (1) molecular formulas (2) structural formulas (3) total number of atoms per molecule (4) total number of bonds per molecule:
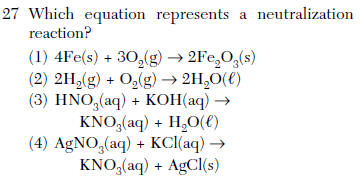 Source: kentchemistry.com
Source: kentchemistry.com
Discusses chemical reactions that occur when acids and bases interact. Which of these elements is. In plants end product alcohol, in animals it is lactate.
 Source: slideplayer.com
Source: slideplayer.com
The overall equation for this reaction is: What is a neutralization reaction? We can write the overall equation for the reaction first.
 Source: yumpu.com
Source: yumpu.com
How many grams of caco3 are needed to neutralize 50 ml of stomach acid at ph = 2.0 completely, if the following equation represents the neutralization reaction? It is called the ionic equation for neutralisation. Acid + base → water + salt.

Acid + base → water + salt. Hcl + naoh h2o + nacl c. A neutralization reaction can be defined as a chemical reaction in which an acid and base quantitatively react together to form a salt and water as products.
 Source: oneclass.com
Source: oneclass.com
(1) base + acid => salt + water (2) base +salt => water + acid (3) salt + acid =>base + water (4) salt + water=> acid + base Net ionic equations for neutralization reactions may include solid acids, solid bases, solid salts, and water. What is the word equation for neutralization in chemistry?
 Source: slideplayer.com
Source: slideplayer.com
Acid + base → water + salt. Neutralization leaves no hydrogen ions in the solution, and the ph of the solution depends on the strength of the acid. We can write the overall equation for the reaction first.
 Source: slidetodoc.com
Source: slidetodoc.com
Consider the reaction between hydrochloric acid and sodium hydroxide; This leaves the ionic equation. A) cah2 b) cao c) cas cas04 8.
 Source: brainly.in
Source: brainly.in
Potassium hydroxide + sulfuric acid → potassium sulfate + water. A base + acid salt + water base + salt water + acid c) salt + acid base + water d) salt + water acid + base hgh 9. Which word equation represents a neutralization reaction?
 Source: studylib.net
Source: studylib.net
Hcl (aq)ca (oh)ho () (aq) a) cah2 b) hcio c) cacl2. Which word equation represents a neutralization reaction? Water + base → salt + acid.
 Source: studylib.net
Source: studylib.net
The products are potassium sulfate and water. When these two chemicals are mixed together, they create a solution of water, or h2o, and potassium sulfate, a salt. The equation for complete oxidation of glucose in anaerobic respiration is glucose >
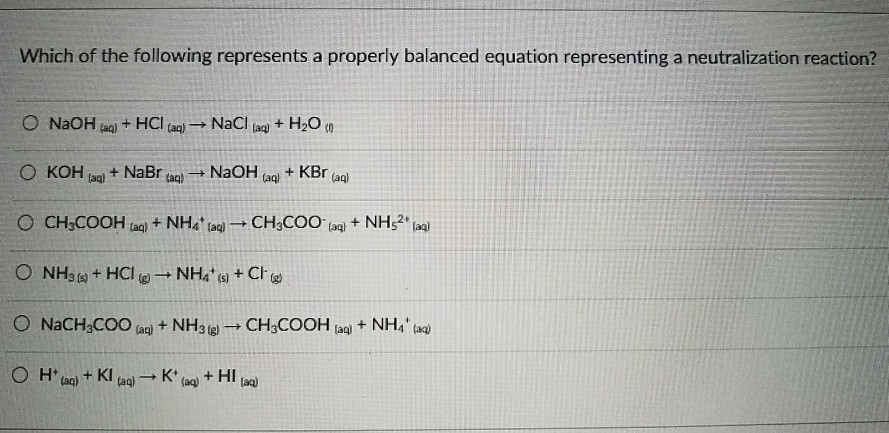
26 which word equation represents a neutralization reaction? (1) base + acid => salt + water (2) base +salt => water + acid (3) salt + acid =>base + water (4) salt + water=> acid + base The ionic equation omits ions which act as spectators so we can split the reactants and the products into ions to see which are the spectators.

What is a neutralization reaction? Which equation represents a neutralization reaction? The word equation for neutralization is acid + base = salt + water.
 Source: chegg.com
Source: chegg.com
Which equation represents a neutralization reaction? Which word equation represents a neutralization reaction? Which word equation represents a neutralization reaction?
 Source: studylib.net
Source: studylib.net
This indicates how strong in your memory this concept is. Sulfuric acid is a strong acid and potassium hydroxide is a strong. The ph value of neutralisation is ph7 because ph1 is a strong acid, ph14 is a strong alkali.
 Source: transtutors.com
Source: transtutors.com
Based on the results of testing colorless solutions with indicators, which solution is most acidic? Which word equation represents a neutralization reaction? In plants end product alcohol, in animals it is lactate.
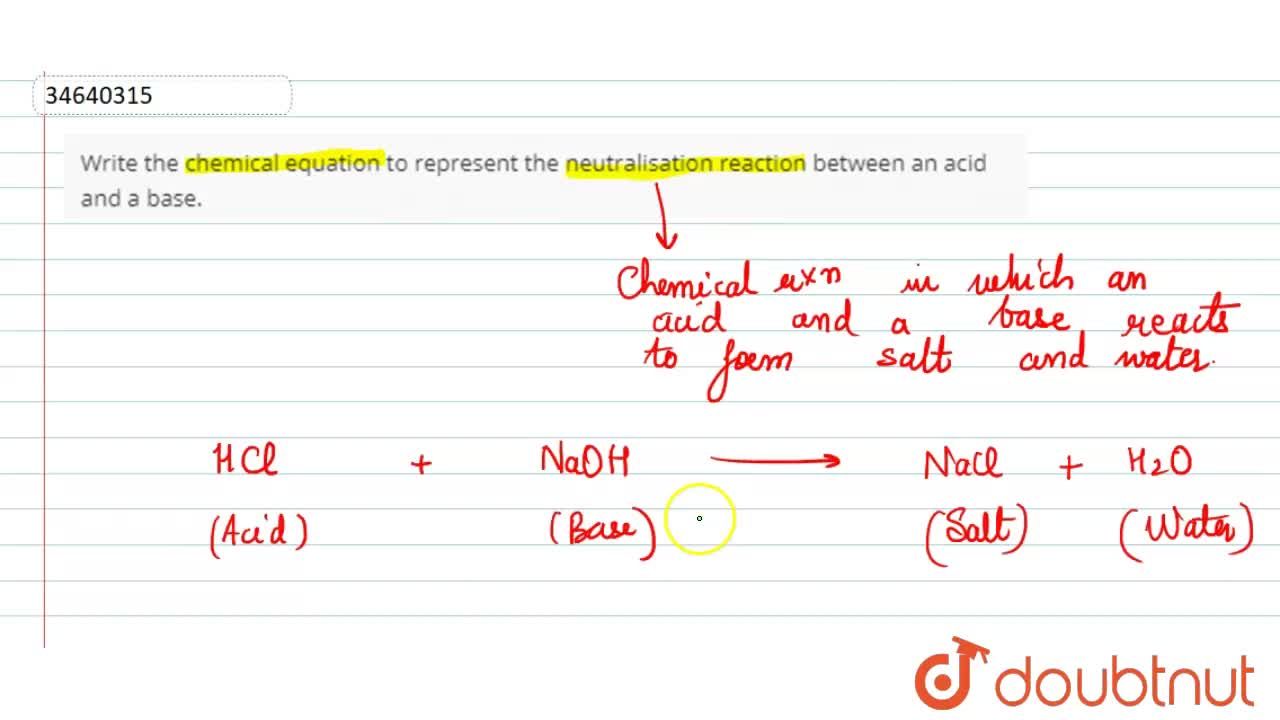 Source: doubtnut.com
Source: doubtnut.com
When these two chemicals are mixed together, they create a solution of water, or h2o, and potassium sulfate, a salt. Base + acid → salt + water an acid solution exactly neutralized a base solution according to the equation acid + base → salt + water. The products are potassium sulfate and water.
 Source: chegg.com
Source: chegg.com
The equation for complete oxidation of glucose in anaerobic respiration is glucose > The ionic equation omits ions which act as spectators so we can split the reactants and the products into ions to see which are the spectators. Potassium hydroxide + sulfuric acid → potassium sulfate + water.
 Source: study.com
Source: study.com
However i to am trying to find the word equation as i have a test tomorrow that i. The word equation for neutralization is acid + base = salt + water. Acid + salt → base + water.
 Source: youtube.com
Source: youtube.com
26 which word equation represents a neutralization reaction? The products are potassium sulfate and water. Ch4 + 2o2 co2 + h2o b.
Also Read :





