Cf4 is nonpolar, with a symmetrical distribution of charge of molecules. As a result, the strongest type of intermolecular interaction between molecules of these substances is the london dispersion force.
Which Type Of Molecule Is Cf4. Which molecule contains the most polar bonds cf4 co2 cn ch4? (1) polar, with a symmetrical distribution of charge. Other types of mixed interactions can also occur. Which type of molecule is cf4?
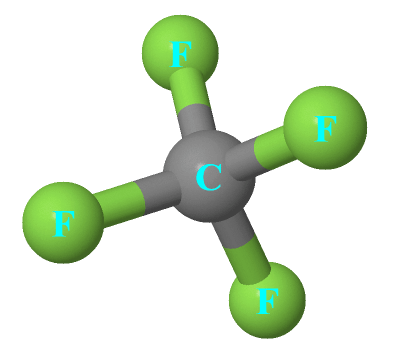 Cf4 Lewis Structure, Molecular Geometry, Polar Or Nonpolar, Bond Angle From topblogtenz.com
Cf4 Lewis Structure, Molecular Geometry, Polar Or Nonpolar, Bond Angle From topblogtenz.com
Related Post Cf4 Lewis Structure, Molecular Geometry, Polar Or Nonpolar, Bond Angle :
A) nonpolar, with a symmetrical distribution of charge. Cf4 is also called tetrafluoromethane in which electronegativity of fluorine is very high; D) polar, with a symmetrical distribution of charge. As with many electron bonds, there are several parts to a tetrahedral molecule.
Reacting geo2 with h2o forms the tetraprotic (four h* ions) acid, h4geo4.on a piece of paper, write.
A) nonpolar, with a symmetrical distribution of charge. It is relatively inert under normal conditions and is a oxygen displacer. Hence, cf4 is nonpolar molecule with a symmetrical distribution of charge. Cf4 is a nonpolar molecule. And the only vector that can be transformed onto itself by a centre of symmetry is the null vector, which in turn means that a dipole moment can only. A) nonpolar, with a symmetrical distribution of charge.
 Source: techiescientist.com
Source: techiescientist.com
Chemical bond a chemical bond is a lasting attraction between atoms, ions or molecules that enables the formation of chemical compounds. Therefore, electron/charge distribution is symmetrical, which also means that the molecule is nonpolar. This set is often in folders with.
 Source: techiescientist.com
Source: techiescientist.com
Cf4 ( carbon tetrafluoride ) is a molecular bond what is chemical bond, ionic bond, molecular bond? Therefore, electron/charge distribution is symmetrical, which also means that the molecule is nonpolar. This set is often in folders with.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Therefore, electron/charge distribution is symmetrical, which also means that the molecule is nonpolar. These planes also generate a centre of symmetry. Therefore, electron/charge distribution is symmetrical, which also means that the molecule is nonpolar.
 Source: topblogtenz.com
Source: topblogtenz.com
It is used as low temperature refrigerant; Cf4 is nonpolar, with a symmetrical distribution of charge of molecules. Cf4 ( carbon tetrafluoride ) is a molecular bond what is chemical bond, ionic bond, molecular bond?
 Source: youtube.com
Source: youtube.com
The main part of the molecule is the atom, which always lies in the center. Which molecule contains the most polar bonds cf4 co2 cn ch4? Determine the electron geometry for each molecule cf4 nf3 of2 h2s.

Cf4 is tetrahedral, so you can consider it to be kind of spherically balanced. Tetrafluoromethane is used with oxygen to etch polysilicon, silicon dioxide, and silicon nitride. The compound is very stable and does not react with acids or hydroxides.
 Source: youtube.com
Source: youtube.com
Cf4 is also called tetrafluoromethane in which electronegativity of fluorine is very high; Cf4 is known as tetrafluoromethane. As a result, the strongest type of intermolecular interaction between molecules of these substances is the london dispersion force.
 Source: dreamstime.com
Source: dreamstime.com
C) nonpolar, with an asymmetrical distribution of charge. Cf4 ( carbon tetrafluoride ) is a molecular bond what is chemical bond, ionic bond, molecular bond? A similar principle applies for cf4.

We can examine which of these forces apply to tetrabromomethane (carbon tetrabromide). A similar principle applies for cf4. The nature of the molecule is polar.
 Source: topblogtenz.com
Source: topblogtenz.com
A) nonpolar, with a symmetrical distribution of charge. Cf4 ( carbon tetrafluoride ) is a molecular bond what is chemical bond, ionic bond, molecular bond? Cf4 is tetrahedral, so you can consider it to be kind of spherically balanced.
 Source: youtube.com
Source: youtube.com
Chemical bond a chemical bond is a lasting attraction between atoms, ions or molecules that enables the formation of chemical compounds. These are stronger than london dispersion forces. What are the characteristics of cf4?
 Source: geometryofmolecules.com
Source: geometryofmolecules.com
Since the four f atoms have the same electronegativity values, there is no bias in electron distribution toward any one. (1) polar, with a symmetrical distribution of charge. We can examine which of these forces apply to tetrabromomethane (carbon tetrabromide).
 Source: clutchprep.com
Source: clutchprep.com
And the only vector that can be transformed onto itself by a centre of symmetry is the null vector, which in turn means that a dipole moment can only. Since the four f atoms have the same electronegativity values, there is no bias in electron distribution toward any one. (2) polar, with an asymmetrical distribution of charge.
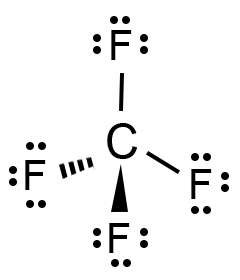 Source: geometryofmolecules.com
Source: geometryofmolecules.com
Cf4 is known as tetrafluoromethane. Cf4 is tetrahedral, so you can consider it to be kind of spherically balanced. Cf4is a molecule with a carbon atom at the center and four fluoridemolecules attached to it.
 Source: clutchprep.com
Source: clutchprep.com
Cf4 is nonpolar, with a symmetrical distribution of charge of molecules. As a result, the strongest type of intermolecular interaction between molecules of these substances is the london dispersion force. Cf4 is also called tetrafluoromethane in which electronegativity of fluorine is very high;
 Source: 123rf.com
Source: 123rf.com
Hence, cf4 is nonpolar molecule with a symmetrical distribution of charge. Which type of molecule is cf 4? These planes also generate a centre of symmetry.
 Source: slideplayer.com
Source: slideplayer.com
Which type of molecule is cf 4? Therefore, electron/charge distribution is symmetrical, which also means that the molecule is nonpolar. Cf4 is also called tetrafluoromethane in which electronegativity of fluorine is very high;

Cf4is a molecule with a carbon atom at the center and four fluoridemolecules attached to it. Molecular geometry for each molecule cf4 nf3 of2 h2s. And the only vector that can be transformed onto itself by a centre of symmetry is the null vector, which in turn means that a dipole moment can only.
 Source: vectorstock.com
Source: vectorstock.com
Which type of molecule is cf4? And the only vector that can be transformed onto itself by a centre of symmetry is the null vector, which in turn means that a dipole moment can only. The molecule has 4 bond pairs no lone pairs of electrons.
 Source: shutterstock.com
Source: shutterstock.com
This set is often in folders with. The cf4 molecule is nonpolar due to its symmetrical tetrahedral structure but the bond present in it polar. The tetrahedral geometry results in a symmetrical arrangement of polar bonds around the carbon atom, as a result, the same charges are distributed within the molecule which helps to cancel out the bond dipole.
Also Read :





