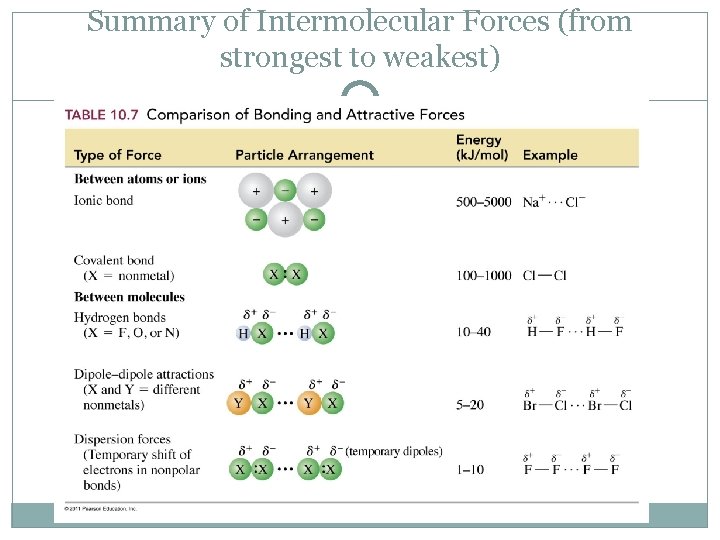
Substance δhvap (kj/mol) argon (ar) 6.3 benzene (c6h6) 31.0 ethanol (c2h5oh) 39.3 water (h2o) 40.8 methane (ch4) 9.2 a) argon b) benzene c) ethanol d) water e) methane The more electronegative a molecule has, the greater the intermolecular force.
Which Substance Has The Weakest Intermolecular Forces. Induced dipole forces are the weakest intermolecular forces and hydrogen bonding is the strongest. The weakest forces are london dispersion forces (#ldf) also known as van der waals (#vdf). Intermolecular attraction a higher boiling point for a liquid indicates a greater attraction between the molecules of that liquid. If a substance has weak intermolecular forces, it has a __________ boiling point, a ___________ heat of vaporization and a ______________ vapor pressure.
 Intermolecular Attractions Bonding And Vsepr Theory Structures Of From slidetodoc.com
Intermolecular Attractions Bonding And Vsepr Theory Structures Of From slidetodoc.com
Related Post Intermolecular Attractions Bonding And Vsepr Theory Structures Of :
What are the strongest to weakest intermolecular forces? There are three different types of intermolecular forces in terms of strength. They exist between all atoms and molecules. Of the molecules that are left, the largest one (c3h8) likely has the strongest london dispersion forces.
/cs5 n,qqbfjr {7 300 o.
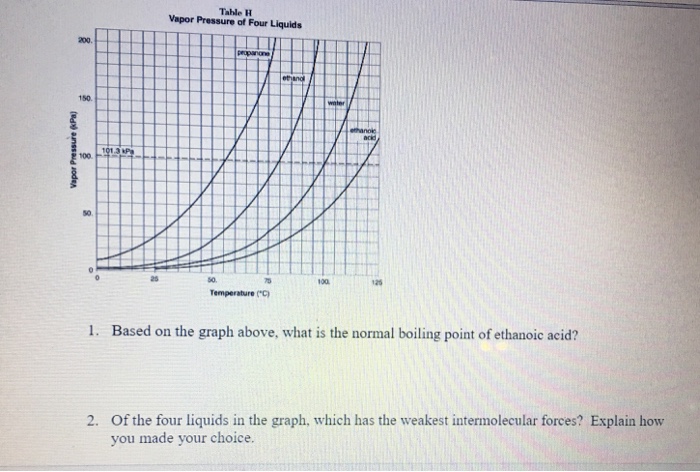
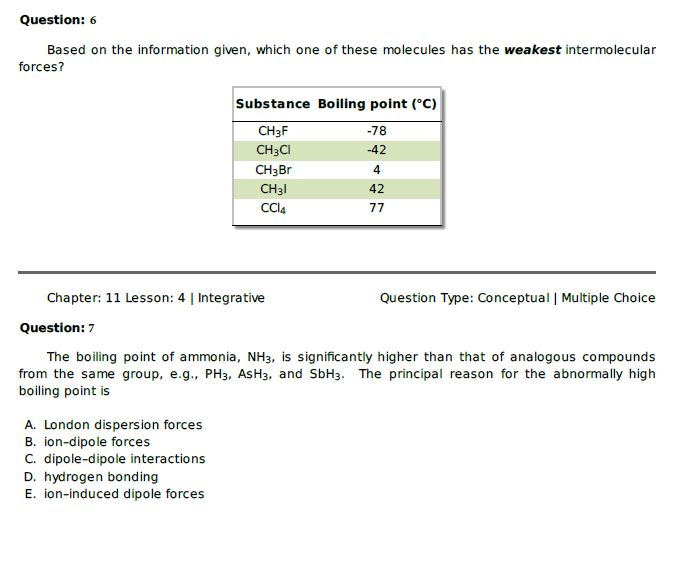
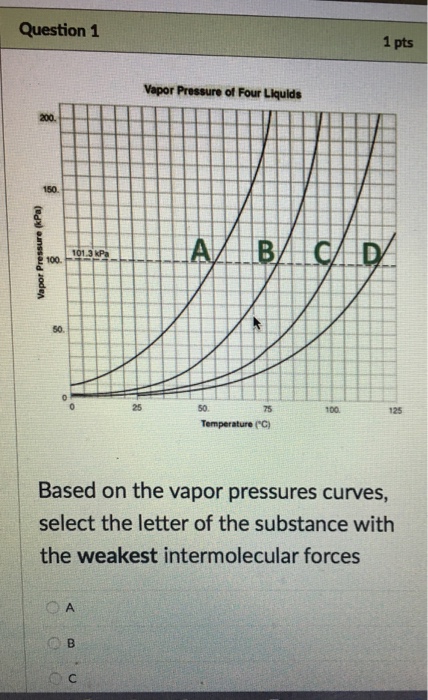
Weak hjgh volatility is hî3h vapor pressure is boiling point is example liquid is Learn this topic by watching intermolecular forces and physical properties. �f) which substance in the graph has the weakest intermolecular forces? Strong hydrogen bonds between water molecules. London dispersion forces are the weakest type of intermolecular bond. Fill in the diagram (with hiqh or low) to show how intermolecular forces influence the volatility, vapor pressure, and boiling point of a substance.

What is the weakest intermolecular force? Among those, the london dispersion forces are the weakest while hydrogen bonding is the strongest of all. Strong hydrogen bonds between water molecules.
 Source: slideplayer.com
Source: slideplayer.com
Which substance has stronger intermolecular forces? If a substance has weak intermolecular forces, it has a __________ boiling point, a ___________ heat of vaporization and a ______________ vapor pressure. Weak hjgh volatility is hî3h vapor pressure is boiling point is example liquid is
 Source: bartleby.com
Source: bartleby.com
London dispersion forces are the weakest type of intermolecular bond. For any given substance, intermolecular forces will be greatest in the solid state and weakest in the gas state. Ch3cooh is the only one of these molecules to have a dipole, and we already decided it has the strongest intermolecular forces.
The weakest forces are london dispersion forces (#ldf) also known as van der waals (#vdf). Which substance has stronger intermolecular forces? Dispersion forces which are present in all molecules.
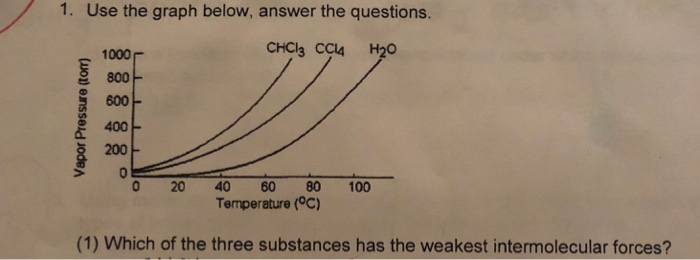
�f) which substance in the graph has the weakest intermolecular forces? There are three different types of intermolecular forces in terms of strength. The smallest (ch4) likely has the weakest intermolecular forces.
 Source: slidetodoc.com
Source: slidetodoc.com
They are the weakest intermolecular force. There are three major intermolecular forces that act on the compound such as; We can tell because the boiling point is less than that of water.
 Source: studylib.net
Source: studylib.net
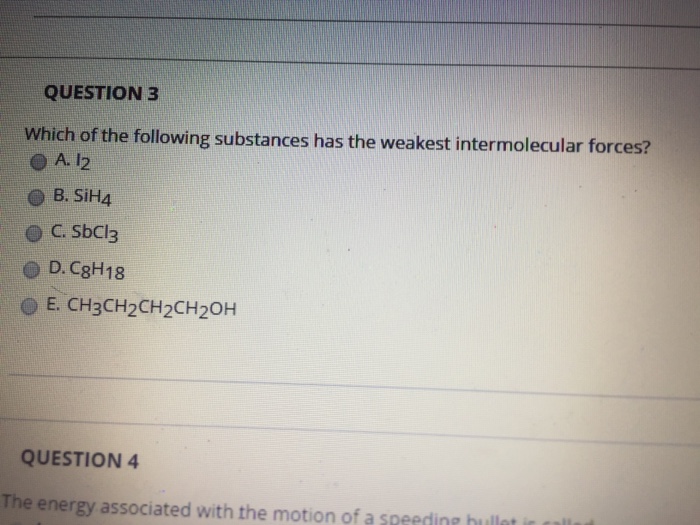
Of the molecules that are left, the largest one (c3h8) likely has the strongest london dispersion forces. Kaypeeoh72z and 4 more users found this answer helpful. The vapor pressure depends only upon the nature of the liquid and the temperature.
 Source: chegg.com
Source: chegg.com
The smallest (ch4) likely has the weakest intermolecular forces. Kaypeeoh72z and 4 more users found this answer helpful. If a substance has weak intermolecular forces, it has a __________ boiling point, a ___________ heat of vaporization and a ______________ vapor pressure.
 Source: chegg.com
Source: chegg.com
As such, liquid and solids have stronger intermolecular forces. The smallest (ch4) likely has the weakest intermolecular forces. Strong hydrogen bonds between water molecules.
 Source: chegg.com
Source: chegg.com
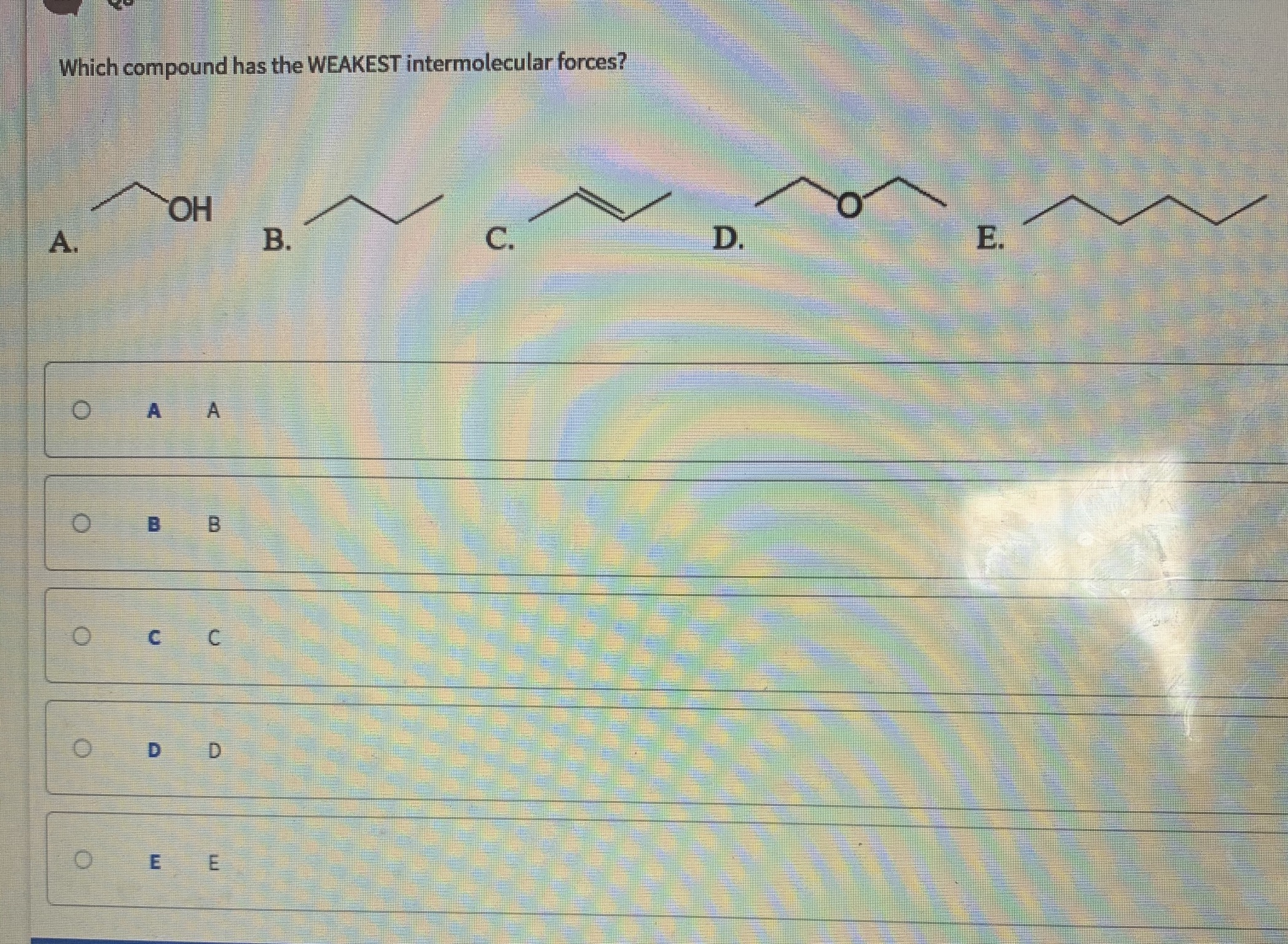
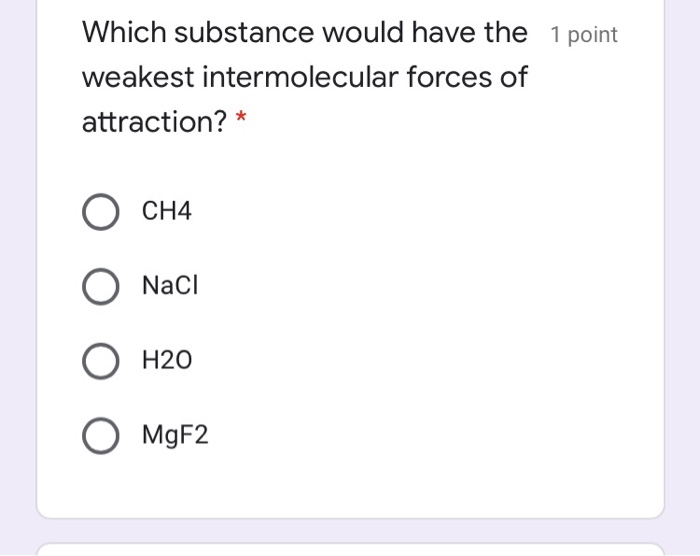
All molecules have this force. For any given substance, intermolecular forces will be greatest in the solid state and weakest in the gas state. Which substance has the weakest intermolecular forces?
 Source: chegg.com
Source: chegg.com
Exhibits hydrogen bonding (strong intermolecular forces) as well as all three van der waals forces — but not as strong as in water. Strong hydrogen bonds between water molecules. If a substance has weak intermolecular forces, it has a __________ boiling point, a ___________ heat of vaporization and a ______________ vapor pressure.
 Source: chegg.com
Source: chegg.com
Water or ammonia are likely to have the strongest forces, while argon, iodine and carbon dioxide are likely to have the weakest forces. If a substance has weak intermolecular forces, it has a __________ boiling point, a ___________ heat of vaporization and a ______________ vapor pressure. O2 is a gas and gas has spread out molecules compared to the other states of matter.
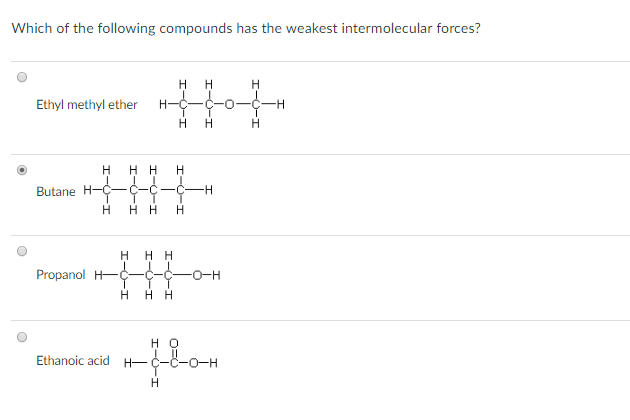 Source: chegg.com
Source: chegg.com
They are the strongest intermolecular force. The vapor pressure depends only upon the nature of the liquid and the temperature. Which substance has stronger intermolecular forces?
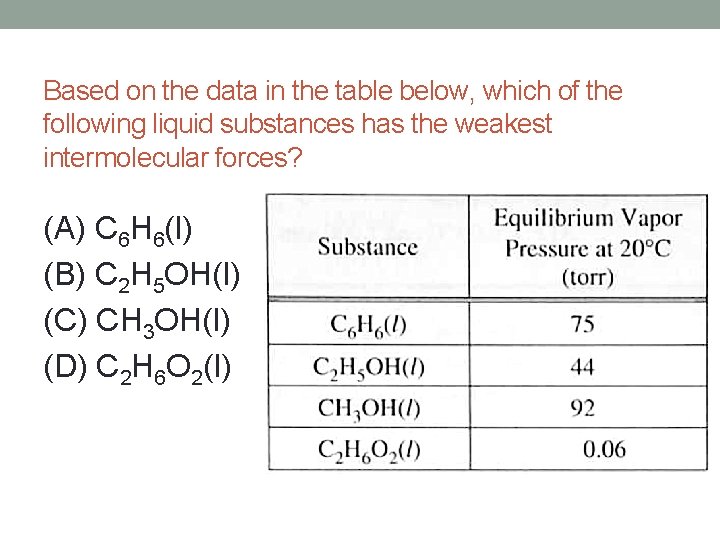 Source: slidetodoc.com
Source: slidetodoc.com
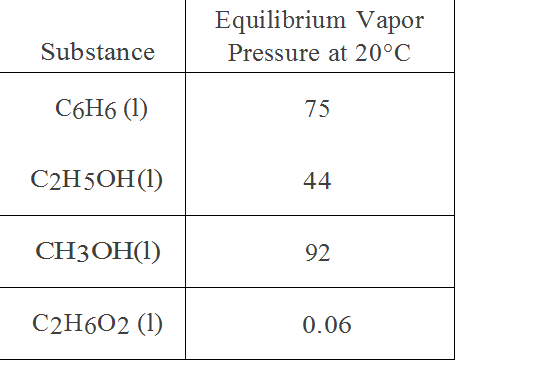
It does not depend upon the amount of liquid. �f) which substance in the graph has the weakest intermolecular forces? Fill in the diagram (with hiqh or low) to show how intermolecular forces influence the volatility, vapor pressure, and boiling point of a substance.
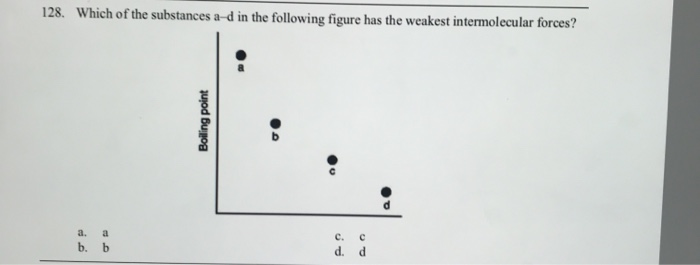 Source: chegg.com
Source: chegg.com
O2 is a gas and gas has spread out molecules compared to the other states of matter. The smallest (ch4) likely has the weakest intermolecular forces. Strong hydrogen bonds between water molecules.
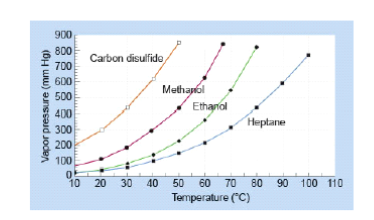 Source: clutchprep.com
Source: clutchprep.com
The smallest (ch4) likely has the weakest intermolecular forces. The weakest forces are london dispersion forces (#ldf) also known as van der waals (#vdf). The more electrons the molecules have, the stronger this force is.
What explains the very high melting and boiling point of water. Among those, the london dispersion forces are the weakest while hydrogen bonding is the strongest of all. There are three major intermolecular forces that act on the compound such as;
 Source: youtube.com
Source: youtube.com
What is the weakest intermolecular force? There are three major intermolecular forces that act on the compound such as; Learn this topic by watching intermolecular forces and physical properties.
 Source: slideplayer.com
Source: slideplayer.com
Learn this topic by watching intermolecular forces and physical properties. Fill in the diagram (with hiqh or low) to show how intermolecular forces influence the volatility, vapor pressure, and boiling point of a substance. What is the weakest intermolecular force?
 Source: slideplayer.com
Source: slideplayer.com
Intermolecular attraction a higher boiling point for a liquid indicates a greater attraction between the molecules of that liquid. The boiling points of ethyl ether and ethanol are 34.6ºc and 78.5ºc respectively. Kaypeeoh72z and 4 more users found this answer helpful.
Also Read :





