Determine the number of protons and electrons in each of the following. The protons and neutrons are in the nucleus at the center of the atom, whilst the electrons how many types of subatomic particles are there?
Which Subatomic Particles Are In The Nucleus. Which particles are found in the nucleus of most atoms? There are three main types of subatomic particles in an atom, protons, neutrons, and electrons. The nucleus of an atom contains which subatomic particles? The structure of a carbon atom, not drawn to scale
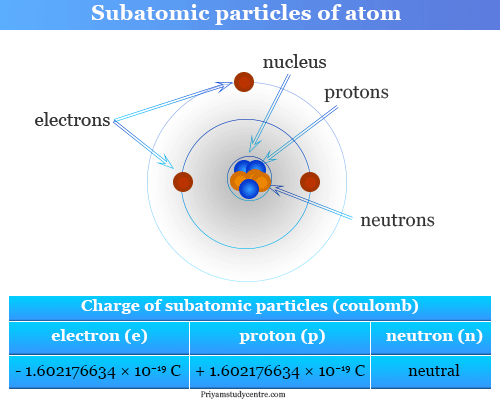 Elementary Particles - Subatomic Particles - List, Mass, Charge From priyamstudycentre.com
Elementary Particles - Subatomic Particles - List, Mass, Charge From priyamstudycentre.com
Related Post Elementary Particles - Subatomic Particles - List, Mass, Charge :
The subatomic particles that are inside the nucleus are protons and neutrons. It is a subatomic particle (symbol n or n 0), which has a neutral charge, and its mass is greater than a proton. The nuclei of most atoms also contain neutrons. Electrons only if an atom has six positively charged subatomic particles, which of the following must it also have in order to become a neutral atom?
Electrons, which have a negative charge, are particles that can found orbiting outside the nucleus of.
Click to see full answer. The center of the atom is called the nucleus. The nucleus of an atom contains which subatomic particles? The three main subatomic particles that form an atom are protons, neutrons, and electrons. The nucleus contains subatomic particles: According to modern atomic theory, an atom has a nucleus, which is its center, or core.
 Source: slideplayer.com
Source: slideplayer.com
The electron travels around out side the nucleus. A third type of subatomic particle, electrons, move around the nucleus. Click to see full answer.
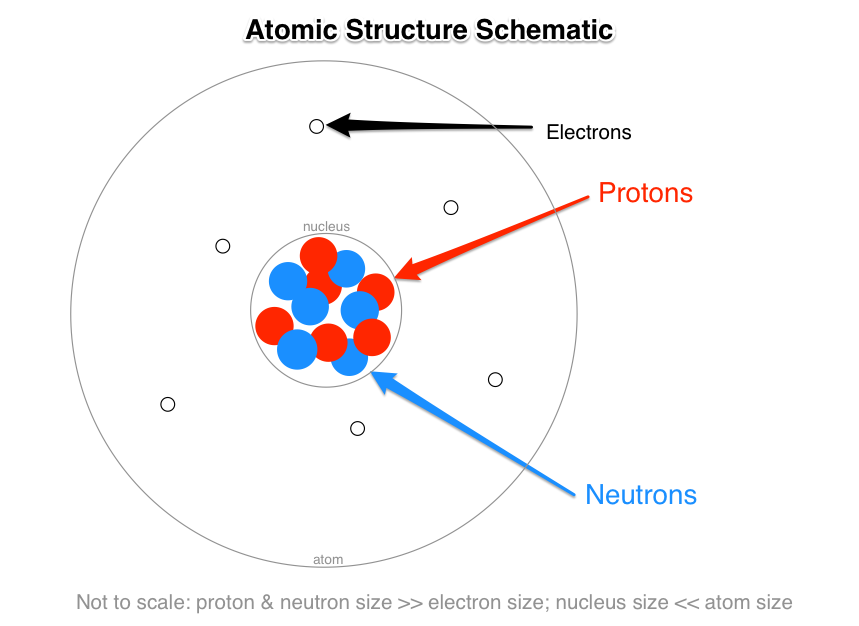 Source: socratic.org
Source: socratic.org
Who invented nucleus in cell? Electrons are called to be the negatively charged subatomic particles Nuclear physics deals with how protons and neutrons arrange themselves in nuclei.
 Source: slideserve.com
Source: slideserve.com
There are two main ways a nucleus can form: As per rutherford’s model of an atom, the positively charged protons are the ones that are present in the atom. Why is an atom neutral?
 Source: youtube.com
Source: youtube.com
Both are together in the center of an atom, called the nucleus. Click to see full answer. The electron travels around out side the nucleus.
 Source: le.ac.uk
Source: le.ac.uk
Subatomic particles are typically located in two places; Chadwick named it neutron in 1932. Atomic nuclei have two kinds of subatomic particles, which are protons and neutrons.
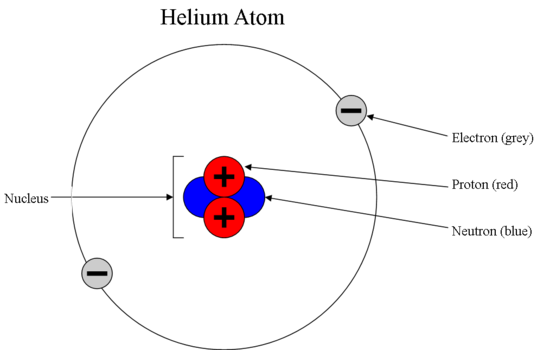 Source: chem.libretexts.org
Source: chem.libretexts.org
Determine the number of protons and electrons in each of the following. Which particles are found in the nucleus of most atoms? The structure of a carbon atom, not drawn to scale
 Source: flexiprep.com
Source: flexiprep.com
It finishes by looking at the existence of isotopes of elements. Electrons are the subatomic particles which revolve around the nucleus of the atom. It has protons and neutrons.
 Source: slideplayer.com
Source: slideplayer.com
The protons have a positive electrical charge and the neutrons have no electrical charge. James chadwick discovered that the nucleus had a new uncharged particle; The protons and electrons b.
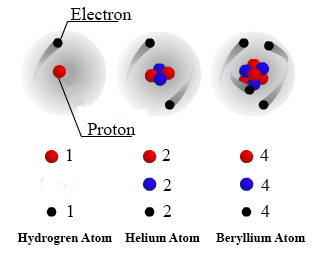 Source: nde-ed.org
Source: nde-ed.org
It finishes by looking at the existence of isotopes of elements. The nucleus of an atom contains which subatomic particles? An atom has hardly any empty space, and the nucleus has a negative charge.
 Source: brainly.com
Source: brainly.com
The nuclei of most atoms also contain neutrons. These two subatomic particles above are located in the nucleus. The nuclei of most atoms also contain neutrons.
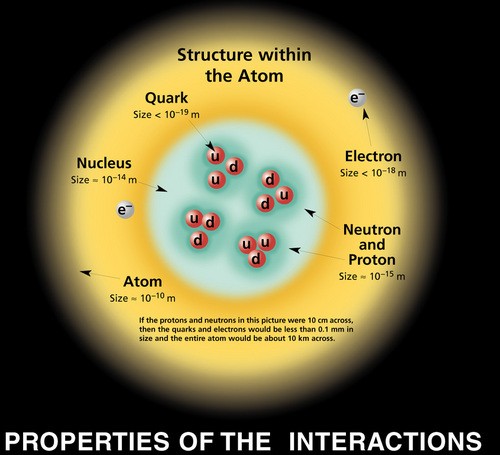 Source: prancer.physics.louisville.edu
Source: prancer.physics.louisville.edu
The center of the atom is called the nucleus. Subatomic particles can usually pass undeflected through an atom because the volume of an atom is composed of a. As per rutherford’s model of an atom, the positively charged protons are the ones that are present in the atom.
 Source: pngwing.com
Source: pngwing.com
The center of the atom is called the nucleus. It finishes by looking at the existence of isotopes of elements. Electrons, which have a negative charge, are particles that can found orbiting outside the nucleus of.
 Source: study.com
Source: study.com
Solved • feb 8, 2020. The subatomic particles of protons and neutrons are found in the nucleus of an atom. Electrons of several different atoms come together to participate in the chemical bonding.
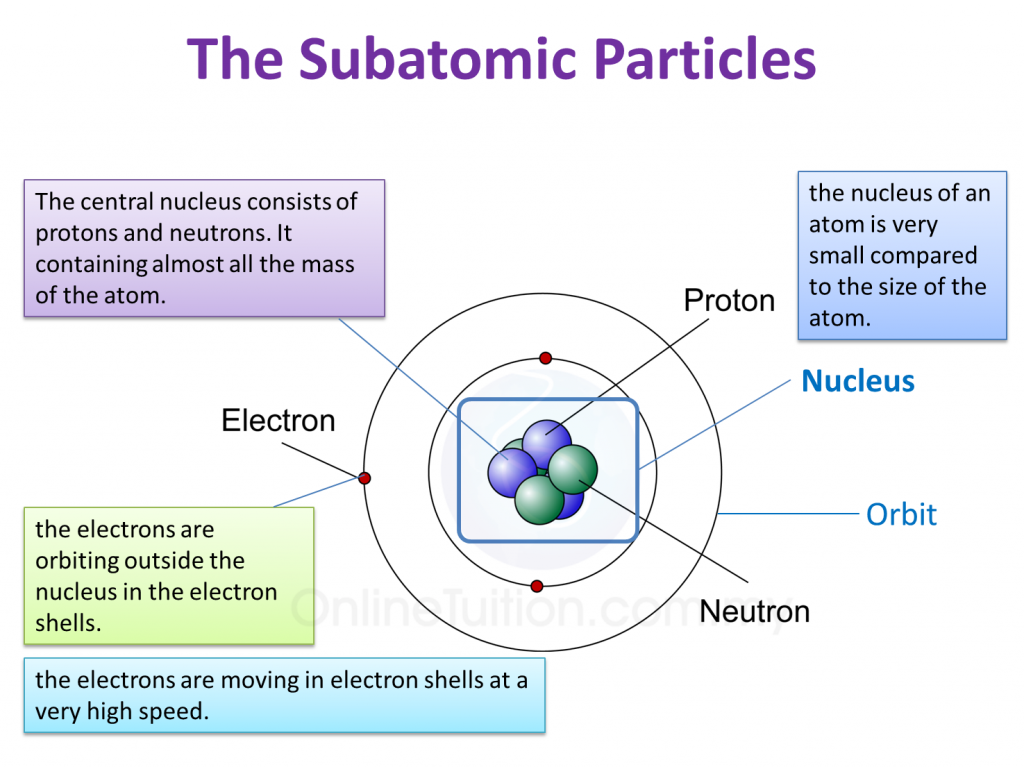 Source: content.myhometuition.com
Source: content.myhometuition.com
Subatomic particles include electrons, the negatively charged, almost massless particles that nevertheless account for most of the size of the atom, and they include the heavier building blocks of the small but very dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons. Electrons of several different atoms come together to participate in the chemical bonding. Some nuclei might also include other types of particle like pions or muons.
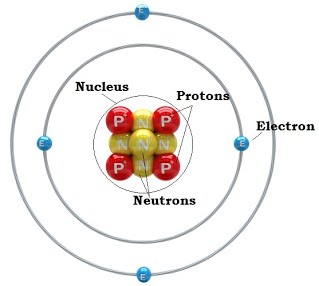 Source: 3galchemy.blogspot.com
Source: 3galchemy.blogspot.com
The structure of a carbon atom, not drawn to scale Why is an atom neutral? The nucleus contains subatomic particles:
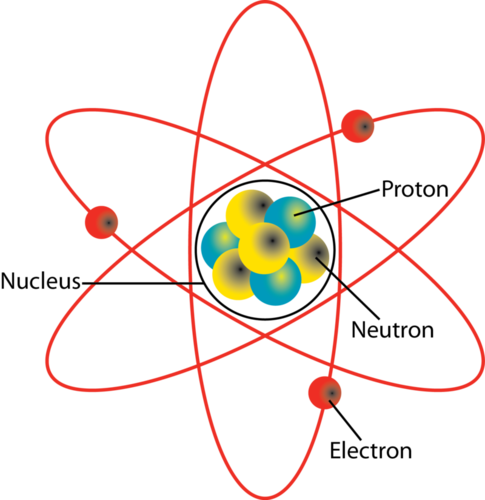 Source: socratic.org
Source: socratic.org
Electrons, which have a negative charge, are particles that can found orbiting outside the nucleus of. Who invented nucleus in cell? Electrons, which have a negative charge, are particles that can found orbiting outside the nucleus of.
 Source: slideplayer.com
Source: slideplayer.com
Solved • feb 8, 2020. The subatomic particles that are inside the nucleus are protons and neutrons. The electron is a subatomic particle that has a negative charge and a negligible mass.
 Source: priyamstudycentre.com
Source: priyamstudycentre.com
A third type of subatomic particle, electrons, move around the nucleus. This page looks briefly at the three subatomic particles we talk about at this level (protons, neutrons and electrons), and then goes on to look at how you work out the numbers of protons and neutrons in the nucleus. The three main subatomic particles that form an atom are protons, neutrons, and electrons.
 Source: sites.google.com
Source: sites.google.com
Who invented nucleus in cell? The nuclei of most atoms also contain neutrons. The electron travels around out side the nucleus.
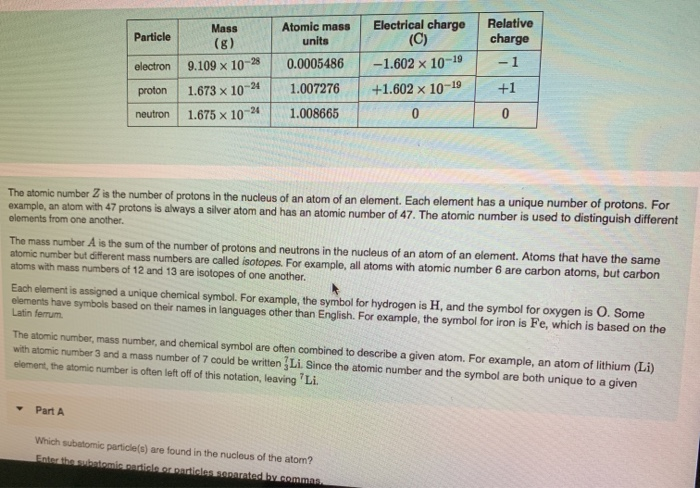 Source: chegg.com
Source: chegg.com
It has protons and neutrons. Both are together in the center of an atom, called the nucleus. This page looks briefly at the three subatomic particles we talk about at this level (protons, neutrons and electrons), and then goes on to look at how you work out the numbers of protons and neutrons in the nucleus.
Also Read :





