) nonmetals generally react by forming covalent bonds (i.e. The fe2+ and fe3+ ions have the same number of protons.
Which Statement Is True Of All Atoms That Are Anions. The oil drop experiment showed the charge of the electron. Anions are atoms with extra electronsc. The adam has more electrons than protons be thie. All are negative, indicating that energy is released.
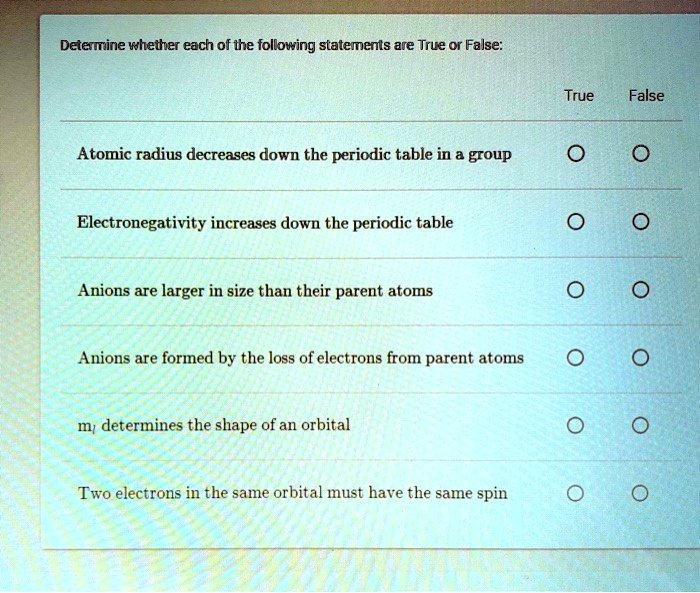 Solved:determine Whether Each Of The Following Statements Are True Or False: True False Atomic Radius Decreases Down The Periodic Table In A Group Electronegativity Increases Down The Periodic Table Anions Are Larger From numerade.com
Solved:determine Whether Each Of The Following Statements Are True Or False: True False Atomic Radius Decreases Down The Periodic Table In A Group Electronegativity Increases Down The Periodic Table Anions Are Larger From numerade.com
Related Post Solved:determine Whether Each Of The Following Statements Are True Or False: True False Atomic Radius Decreases Down The Periodic Table In A Group Electronegativity Increases Down The Periodic Table Anions Are Larger :
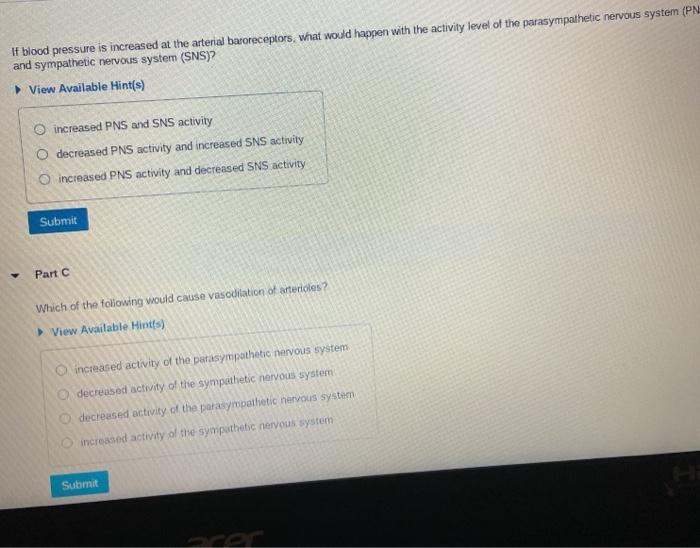
These ions are atoms that gain or lose electrons, giving them a net positive or negative charge. Select all the true statements. Which statement is true of all atoms that are anions? (b) the atom has more protons than electrons.
See what gk and i wrote below.
Which statement is true of all atoms that are anions?(a) the atom has more electrons than protons.(b) the atom has more protons than electrons.(c) the atom has fewer protons than does a neutral atomof the same element.(d) the atom has more neutrons than protons. Which statement is true of all atoms that are anions? B) the atom has more protons than electrons. A) the atom has more electrons than protons. Anions result when atoms gain an electron. Which statement is true of all atoms that are anions?
 Source: chegg.com
Source: chegg.com
Which of the following statements correctly describes any. (a) the atom has more electrons than protons. A) the atom has more electrons than protons.
 Source: numerade.com
Source: numerade.com
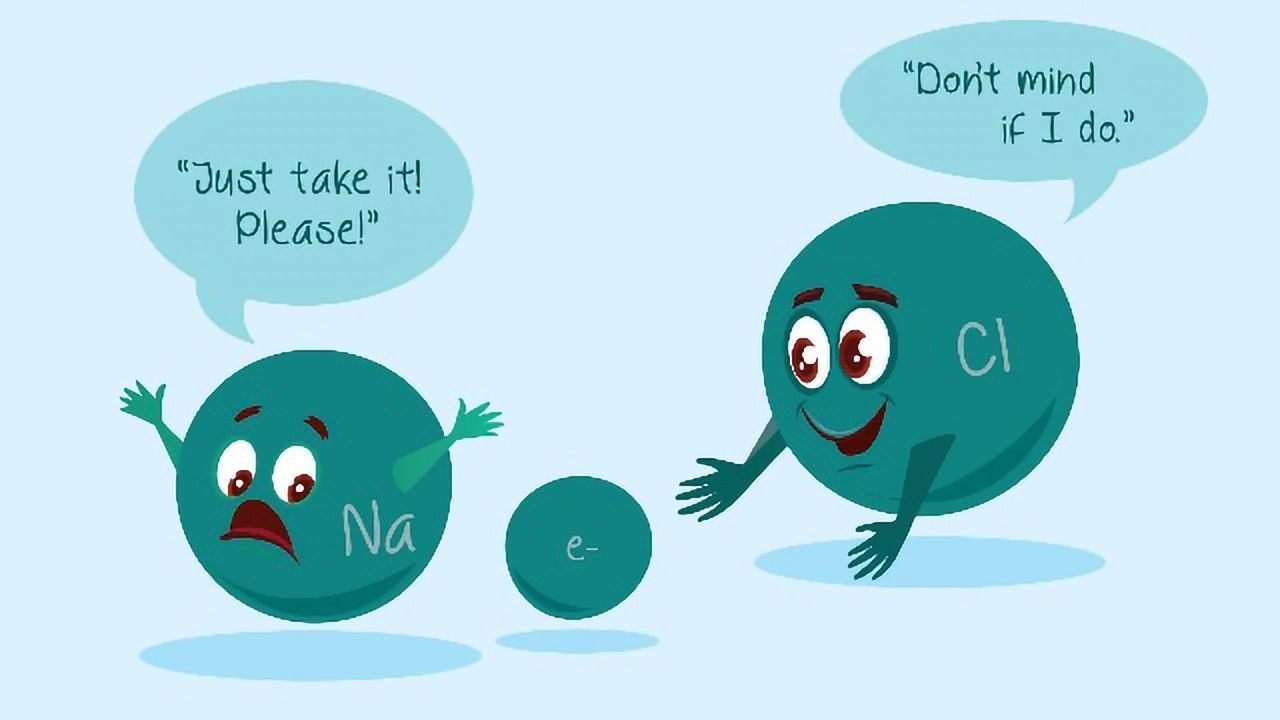
One atom transfers an electron to another atom. These ions are atoms that gain or lose electrons, giving them a net positive or negative charge. ) halogen atoms have the highest ionization energies amongst all groups in the periodic table.
 Source: numerade.com
Source: numerade.com
Which statement is true of all atoms that are anions? When an atom gains an electron, it becomes a cation. ) nonmetals generally react by forming covalent bonds (i.e.
 Source: numerade.com
Source: numerade.com
One atom transfers an electron to another atom. (b) the atom has more protons than electrons. Select all the true statements.
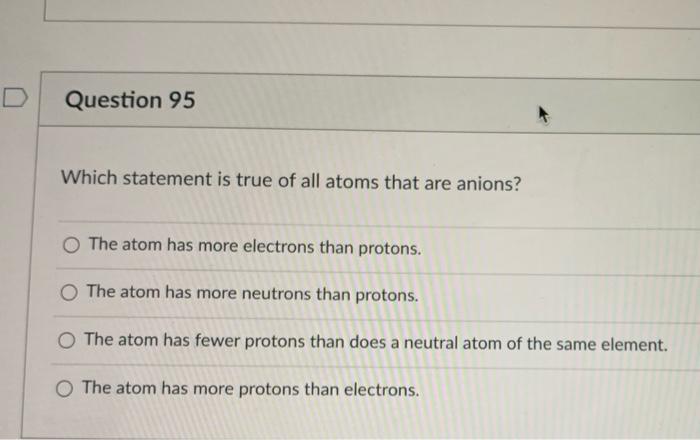
The adam has more neutrons and protons. Anions are atoms or radicals (groups of atoms), that have gained electrons. All are negative, indicating that energy is released.
 Source: slidetodoc.com
Source: slidetodoc.com
Adam has more protons than electrons. Cations are atoms missing electronse. ) metals are good conductors of heat and electricity.

Which of the following statements correctly describes any chemical reaction that has reached equilibrium? The atom has more protons than electrons. Combining cations and anions to form binary inorganic compounds is simple.
 Source: studylib.net
Source: studylib.net
The adam has more electrons than protons be thie. Which statement is true of all atoms that are anions? Which of the following statements correctly describes any.
 Source: numerade.com
Source: numerade.com
(b) the atom has more protons than electrons. So let�s get into the question. The existence of unpaired electrons in the valence shell
 Source: numerade.com
Source: numerade.com
The cu^+ and cu^2+ ions have the same number of electrons. All are negative, indicating that energy is released. ) metals are good conductors of heat and electricity.
 Source: numerade.com
Source: numerade.com
The atom has more electrons than protons. Anions are atoms or radicals (groups of atoms), that have gained electrons. The atom has more protons than electrons.
 Source: technologynetworks.com
Source: technologynetworks.com
Nonmetals tend to gain electrons, forming anions that have a net negative charge. Select all the true statements. (c) the atom has fewer protons than does a neutral atom of the same element.
 Source: chegg.com
Source: chegg.com
Ions are atoms that have gained or lost electron. The atom has more electrons than protons. One atom transfers an electron to another atom.
 Source: chegg.com
Source: chegg.com
The existence of unpaired electrons in the valence shell Ions are atoms that have gained or lost electron. When an atom gains an electron, it becomes a cation.
 Source: chegg.com
Source: chegg.com
The atom has more electrons than protons. The atom has more electrons than protons. Three different atoms or atomic anions with 18 electrons:
 Source: studylib.net
Source: studylib.net
See, the adam has fewer protons than does a neutral atom of the same element or d. See what gk and i wrote below. What is true about an ionic compound?
 Source: chegg.com
Source: chegg.com
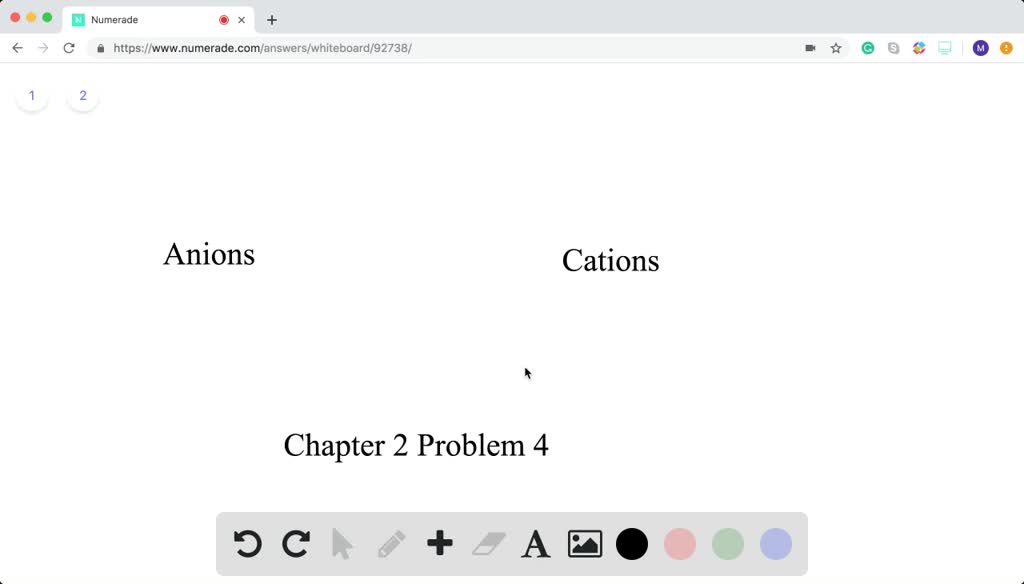
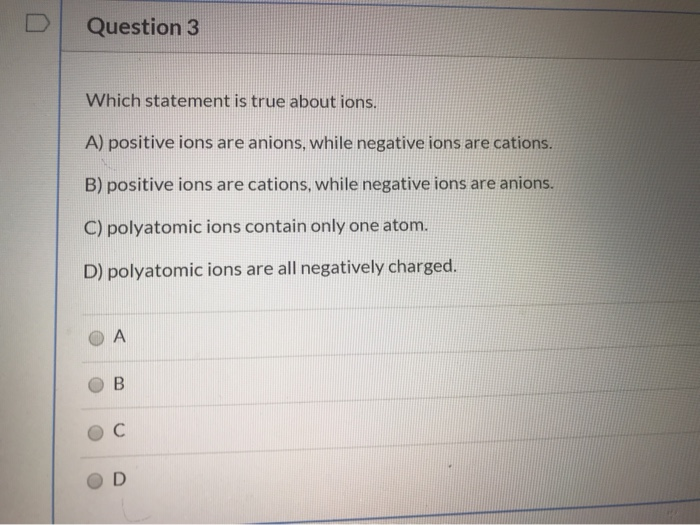
Anions carry negative charge over them. If the atom is neutral then the number of protons will be equal to the number of electrons. So chapter two problem for which statement is true of all atoms that are an ions so negatively charged.
 Source: numerade.com
Source: numerade.com
The atomic number (z) indicates the number of protons in an atom of an element. When an atom gains an electron, it becomes a cation. The atom has more protons than electrons.
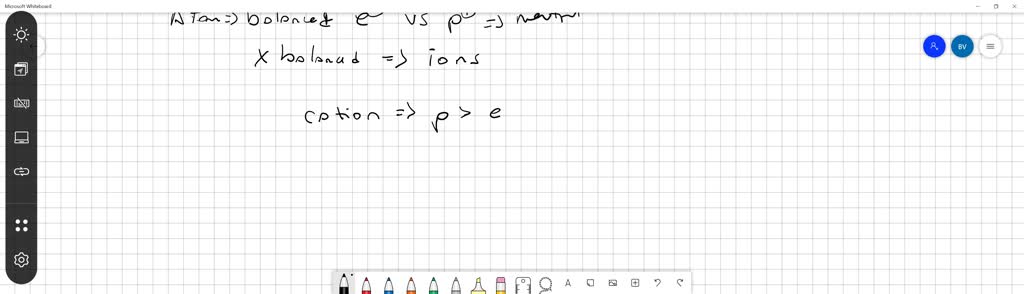 Source: numerade.com
Source: numerade.com
We know somewhat about atoms, yet not it�s exact shape.we know that atoms have a nucleus: The atom has fewer protons than does a neutral atom of the same element.d. The atom has more electrons than protons.b.
 Source: chegg.com
Source: chegg.com
Sort the subatomic particles according to their masses. Which statement is true of all atoms that are anions?(a) the atom has more electrons than protons.(b) the atom has more protons than electrons.(c) the atom has fewer protons than does a neutral atomof the same element.(d) the atom has more neutrons than protons. The atom has more electrons than protons.
Also Read :