Which statement best describes a relationship between mitochondria and chloroplasts? The theoretical yield is calculated from the amount of the limiting reactant present.
Which Statement Correctly Describes The Relationship Between Reactant And Yield. Relationship between coupon rate and its yield to maturity is as such that if coupon rate is lower than its yield to maturity or required rate of return then bond is valued at less than face value. The actual yield is calculated from the amount of the limiting reactant, and the. All reactants and products are gases. A 13.00 g sample of citric acid reacts with an excess of baking soda as shown in the equation.
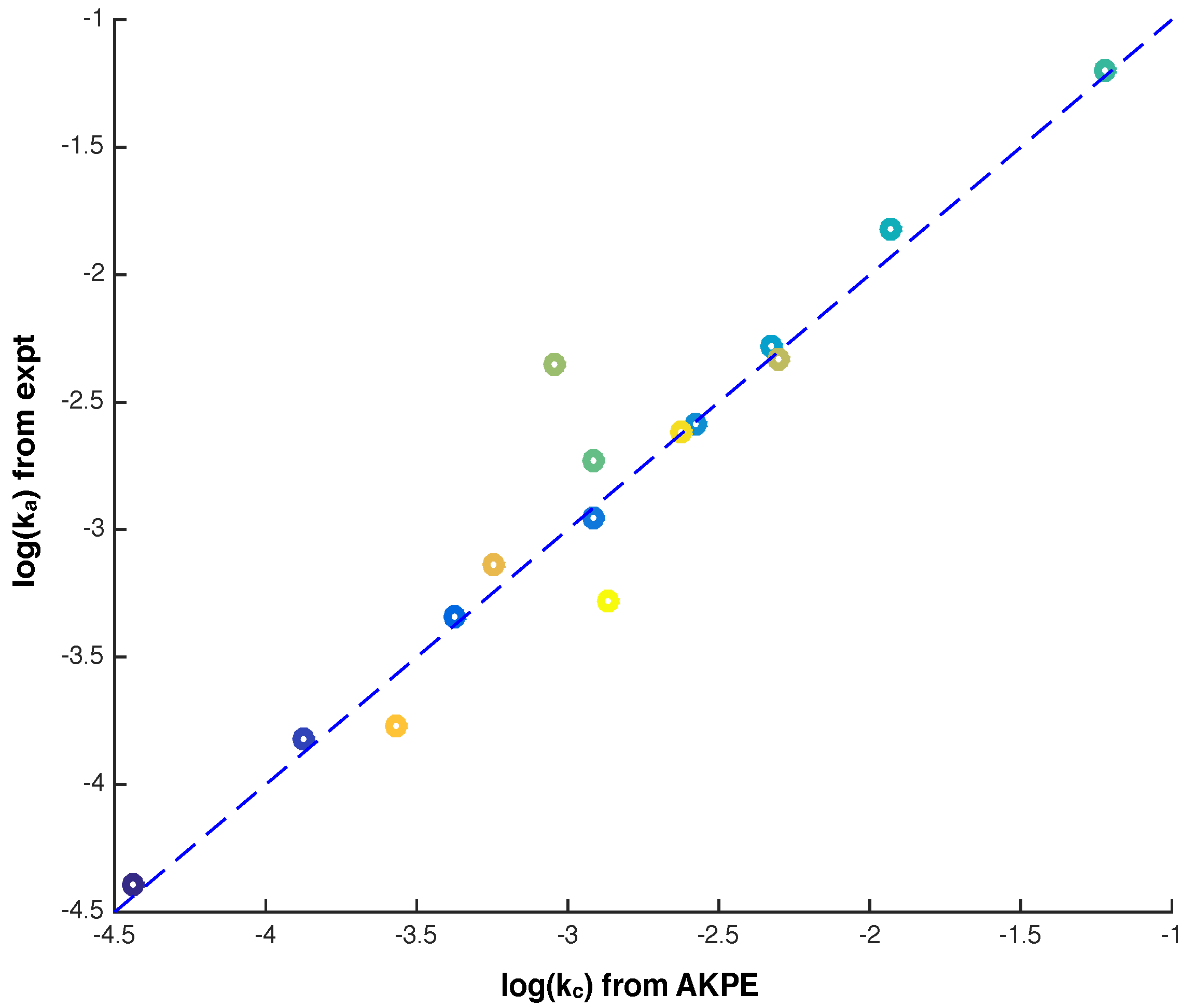 Electronics | Free Full-Text | Simulation Of Biochemical Reactions With Ann-Dependent Kinetic Parameter Extraction Method | Html From mdpi.com
Electronics | Free Full-Text | Simulation Of Biochemical Reactions With Ann-Dependent Kinetic Parameter Extraction Method | Html From mdpi.com
Related Post Electronics | Free Full-Text | Simulation Of Biochemical Reactions With Ann-Dependent Kinetic Parameter Extraction Method | Html :
1 13% of cells germinate without the addition of extra gibberellin. Determine the percent yield if 6.97 grams of ammonia is produced from the reaction of 6.22 grams of nitrogen with excess hydrogen. The theoretical yield is calculated from the amount of the limiting reactant present. Which statement correctly describes the relationship between thermal energy and particle movement?
Equilibrium is reached in a very short time.
2 gibberellin concentrations of greater than 0.5 mmol dm−3 do not result in seed germination greater than 84%. Side of reaction with fewer moles forward reaction a reactant exothermic 6.97 g nh 3 is the actual yield 6.22 g n 2 is the amount of reactant. Which statement best describes a relationship between mitochondria and chloroplasts? Which statement is correct for a reversible reaction when ? All reactants and products are gases.
 Source: chegg.com
Source: chegg.com
A 13.00 g sample of citric acid reacts with an excess of baking soda as shown in the equation. Which statement correctly describes one similarity between photosynthesis and cellular respiration? A catalyst increases the rate of the reaction by lowering the activation energy.
 Source: chegg.com
Source: chegg.com
Which statement correctly describes the relationship between reactant and yield? 1 13% of cells germinate without the addition of extra gibberellin. A review of the above reactions reveals that there is a 1:1 relationship between the aluminum containing reactant and the aluminum containing product in all cases.

Determine the percent yield if 6.97 grams of ammonia is produced from the reaction of 6.22 grams of nitrogen with excess hydrogen. At equilibrium, the rate of the forward reaction is much higher than the rate of the backward reaction. The actual yield depends on the reaction conditions, but the theoretical yield varies only with reactant amounts.
If 200.0 g of copper(ii) sulfate react with an excess of zinc metal, what is the theoretical yield of copper? A review of the above reactions reveals that there is a 1:1 relationship between the aluminum containing reactant and the aluminum containing product in all cases. A 13.00 g sample of citric acid reacts with an excess of baking soda as shown in the equation.
 Source: quizlet.com
Source: quizlet.com
3 concentration of gibberellin has the biggest effect on seed germination between At equilibrium, the rate of the forward reaction is much higher than the rate of the backward reaction. 2) excess reactant is the one that is in greater proportion than the theoretical ratio.
 Source: yumpu.com
Source: yumpu.com
Relationship between coupon rate and its yield to maturity is as such that if coupon rate is lower than its yield to maturity or required rate of return then bond is valued at less than face value. Which statement correctly describes the relationship between reactant and yield? Read the following article, which introduces the concept of treasury bills and the relationship between their yield and price.
 Source: brainly.com
Source: brainly.com
Which energy is primarily responsible for phenomena such as the movement of large plates and volcanoes? The theoretical yield is calculated from the amount of the limiting reactant present. 15 the graph shows how the yield of product in a reversible reaction changes as the temperature and pressure are changed.
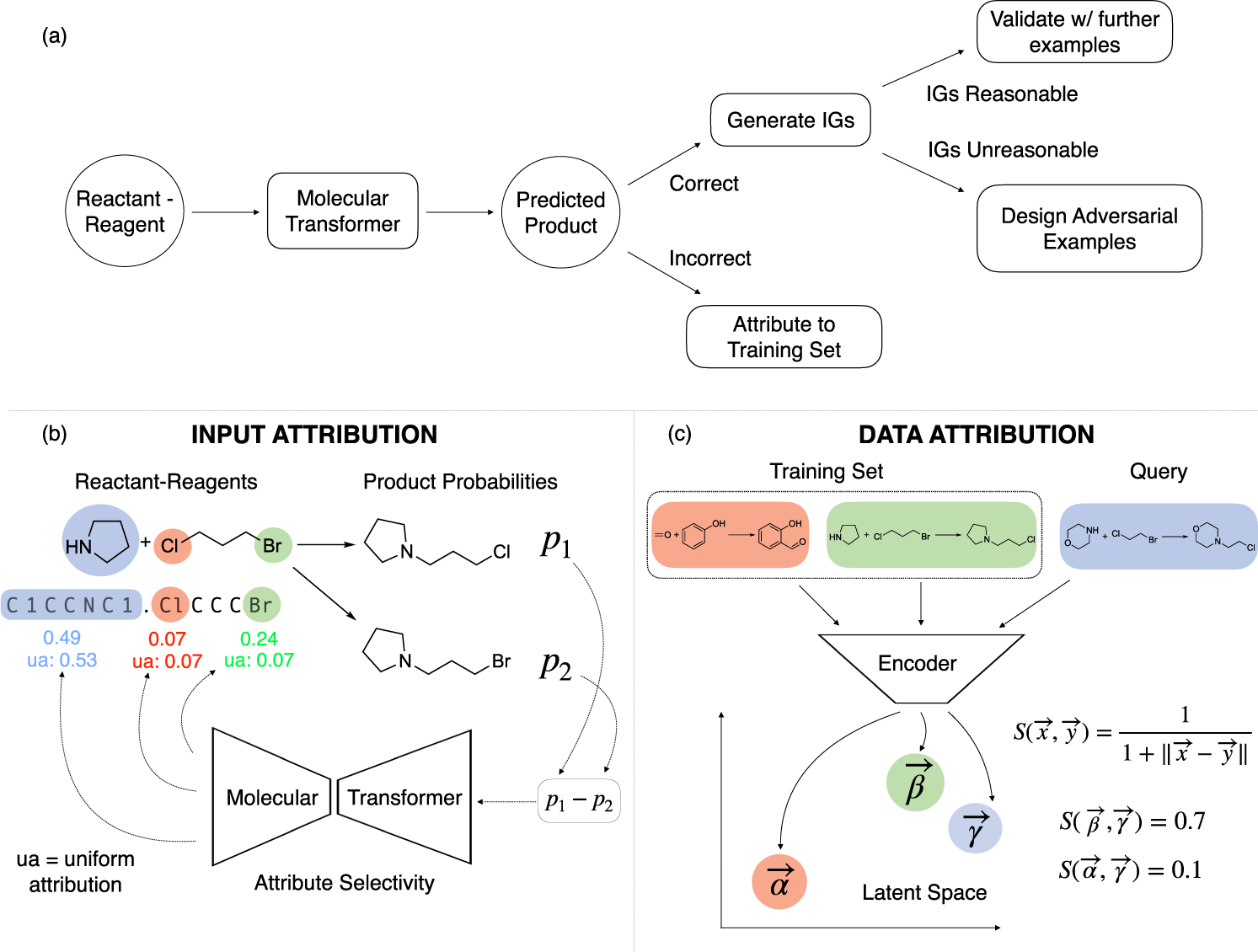 Source: nature.com
Source: nature.com
15 the graph shows how the yield of product in a reversible reaction changes as the temperature and pressure are changed. At equilibrium, the rate of the forward reaction is much higher than the rate of the backward reaction. Which statement best describes a relationship between mitochondria and chloroplasts?
 Source: brainly.com
Source: brainly.com
Which statement correctly describes the relationship between reactant and yield? �the theoretical yield is calculated from the amount of the limiting reactants present�. Which statement correctly describes the relationship between reactant and yield?
 Source: quizlet.com
Source: quizlet.com
A 13.00 g sample of citric acid reacts with an excess of baking soda as shown in the equation. Determine the percent yield if 6.97 grams of ammonia is produced from the reaction of 6.22 grams of nitrogen with excess hydrogen. A catalyst does not affect the thermodynamic parameters of a reaction such as enthalpy, entropy, gibbs energy, and equilibrium constant.
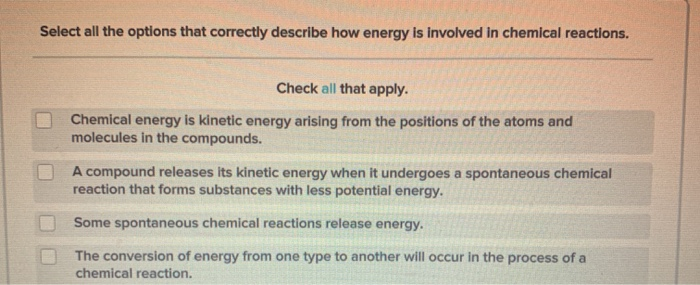 Source: chegg.com
Source: chegg.com
A catalyst does not affect the thermodynamic parameters of a reaction such as enthalpy, entropy, gibbs energy, and equilibrium constant. The actual yield is calculated from the amount of the excess reactant present. E number of effective collisions between reactant particles is increased, he rate of the reverse reaction is increased.
 Source: mdpi.com
Source: mdpi.com
The actual yield is calculated from the reactant amounts, but the theoretical yield must be measured for each instance of a reaction. The actual yield is calculated from the amount of the limiting reactant present. 3 concentration of gibberellin has the biggest effect on seed germination between
 Source: chegg.com
Source: chegg.com
The actual yield depends on the reaction conditions, but the theoretical yield varies only with reactant amounts. 15 the graph shows how the yield of product in a reversible reaction changes as the temperature and pressure are changed. E number of effective collisions between reactant particles is increased, he rate of the reverse reaction is increased.
 Source: brainly.com
Source: brainly.com
6.97 g nh 3 is the actual yield 6.22 g n 2 is the amount of reactant. As thermal energy increases, there is less particle movement b. Which statement correctly describes the actual yield and the theoretical yield of a reaction?
 Source: chegg.com
Source: chegg.com
Notice that two quantities are given in the problem statement. The theoretical yield is calculated from the amount of the limiting reactant present. The actual yield is calculated from the amount of the limiting reactant present.
 Source: bartleby.com
Source: bartleby.com
Read the following article, which introduces the concept of treasury bills and the relationship between their yield and price. Which statement correctly describes one similarity between photosynthesis and cellular respiration? Reactants products yield of product pressure 100 °c 300°c which row is correct for this reversible reaction?
 Source: brainly.com
Source: brainly.com
As catalyst increases the reaction rate by lowering the activation energy, so, option a is correct. The actual yield depends on the reaction conditions, but the theoretical yield varies only with reactant amounts. Which statement correctly describes one similarity between photosynthesis and cellular respiration?
 Source: brainly.com
Source: brainly.com
This can be used to Side of reaction with fewer moles forward reaction a reactant exothermic The theoretical yield is calculated from the amount of the limiting reactant present.
 Source: chegg.com
Source: chegg.com
1 13% of cells germinate without the addition of extra gibberellin. + used to separate one reactant or product from another. Which statement correctly describes the actual yield and the theoretical yield of a reaction?
 Source: quizlet.com
Source: quizlet.com
Then answer the question that follows. �the theoretical yield is calculated from the amount of the limiting reactants present�. E number of effective collisions between reactant particles is increased, he rate of the reverse reaction is increased.
Also Read :





