What is the magnetic property of c2? The electronic configuration of no will be (ii) no 2.
Which Species Is Diamagnetic. All the electrons in a carbon monoxide molecule are paired and thus it’s diamagnetic in nature. For diamagnetic behaviour number of unpaired electrons should be zero. If all the electrons in the atom or molecule are paired then the substance is diamagnetic. Therefore due to absence of you do have a sense of unfair electron as to will be as you will be died magnetic in nature.
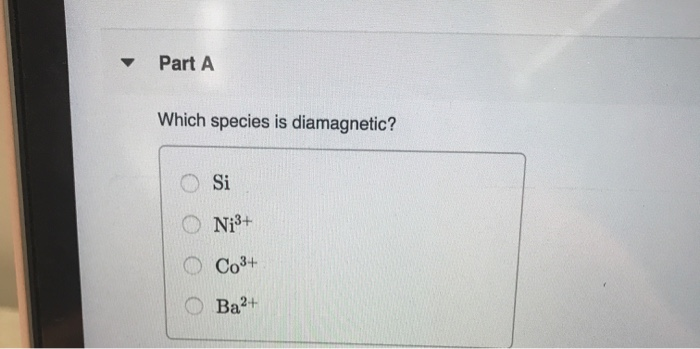 Solved Part A Which Species Is Diamagnetic? Si On: 3+ Осo3+ | Chegg.com From chegg.com
Solved Part A Which Species Is Diamagnetic? Si On: 3+ Осo3+ | Chegg.com From chegg.com
Related Post Solved Part A Which Species Is Diamagnetic? Si On: 3+ Осo3+ | Chegg.com :
Overall 4 species are paramagnetic including no, no 2. And you have known the answer to this chemistry question. In chemistry, the thumb rule is used to determine whether the particle atom, ion or molecule is paramagnetic or diamagnetic. Total number of electrons = 7+8 = 15.
Total number of electrons = 7+8+8 = 23.
Please log inor registerto add a comment. Due to its weak magnetic property, the effects of. The electronic configuration of no will be (ii) no 2. And beard electrons present here. No + molecule is therefore diamagnetic. Give the complete electron configuration of cobalt, co, the complete or abbreviated electron configuration, of co2+, and co3+, and predict the number of unpaired electrons in all three.
 Source: clutchprep.com
Source: clutchprep.com
The electronic configuration of no 2 will be Water has no unpaired electrons and is thus diamagnetic. Since the 4d and 5s subshells are entirely filled, there are no unpaired electrons and sn2+ by definition is diamagnetic.
 Source: toppr.com
Source: toppr.com
But, in materials that show other magnetic properties such as paramagnetism and ferromagnetism, the effect of diamagnetism is negligible. And beard electrons present here. If the the bond order is fractional, species are paramagnetic.
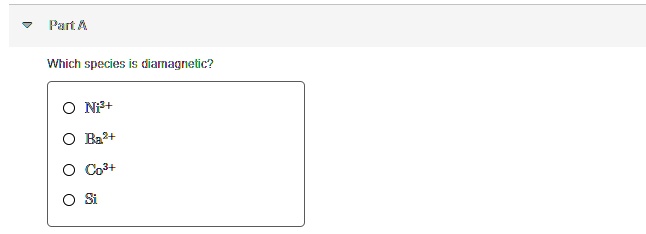 Source: numerade.com
Source: numerade.com
The electronic configuration of no will be (ii) no 2. Are filled accordingly whereas in the case of molecules which consists of minimum two atoms we will be dealing with molecular. For diamagnetic behaviour number of unpaired electrons should be zero.
 Source: youtube.com
Source: youtube.com
The os centre is in a +2 oxidation state. Keep in mind that if we have only one atom, then we are talking about atomic orbitals and its subshells, shells etc. Therefore the answer is option (d).

Using mo theory, predict which of the following species is diamagnetic: The electronic configuration of no 2 will be For diamagnetic behaviour number of unpaired electrons should be zero.
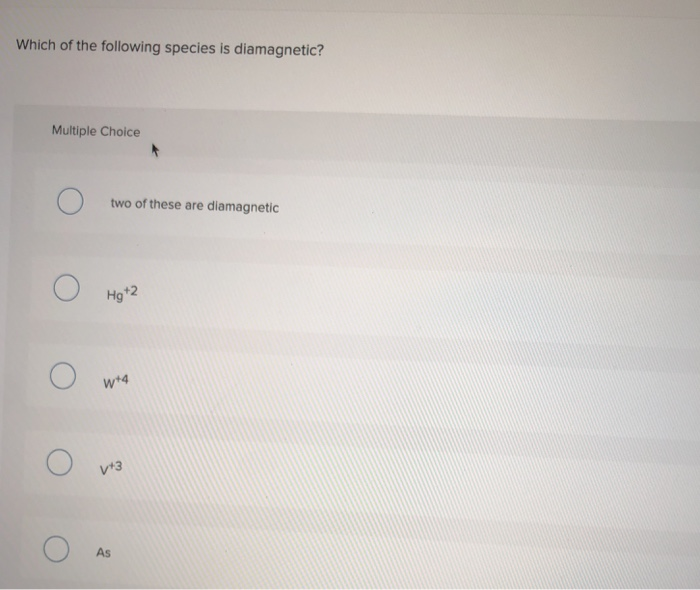 Source: chegg.com
Source: chegg.com
In chemistry, the thumb rule is used to determine whether the particle atom, ion or molecule is paramagnetic or diamagnetic. Generally, all materials have the diamagnetic properties, making a weak contribution to the magnetic behaviour of the material when subjected to an external magnetic field. Water has no unpaired electrons and is thus diamagnetic.
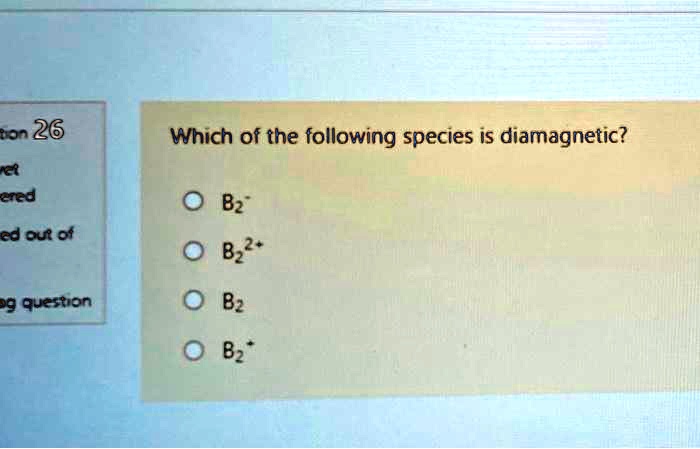 Source: numerade.com
Source: numerade.com
So according to the option, option b eat correct answer ups and be it correct answer. In chemistry, the thumb rule is used to determine whether the particle atom, ion or molecule is paramagnetic or diamagnetic. The neutral oxygen is paramagnetic according to mo theory because it.
 Source: toppr.com
Source: toppr.com
Please log inor registerto add a comment. This is the best answer based on feedback and ratings. Overall 4 species are paramagnetic including no, no 2.
 Source: brainly.in
Source: brainly.in
However if one or more molecular orbital are singly occupied it is paramagnetic e.g. Paramagnetic species in the following is clo2 only. Therefore due to absence of you do have a sense of unfair electron as to will be as you will be died magnetic in nature.
 Source: clutchprep.com
Source: clutchprep.com
If the electronic configuration of a molecule has only paired or spin paired electrons, then that molecule is said to be diamagnetic. The os centre is in a +2 oxidation state. ← prev questionnext question →.

The neutral oxygen is paramagnetic according to mo theory because it. Total number of electrons = 7+8+8 = 23. Chemical bonding and molecular structure.

What is the magnetic property of c2? Here the molecule c2 has all paired electrons in its electronic configuration. Determine the number of unpaired electrons for argon.
 Source: brainly.in
Source: brainly.in
To know the magnetic character of molecules we can use mo diagram. Cn+ о 1 and 3 only o 1 only o 2 and 3 only o 1, 2, and 3. Therefore due to absence of you do have a sense of unfair electron as to will be as you will be died magnetic in nature.
 Source: doubtnut.com
Source: doubtnut.com
On page 71 of tbr chem, it states this about diamagnetic species: Here the molecule c2 has all paired electrons in its electronic configuration. To know the magnetic character of molecules we can use mo diagram.
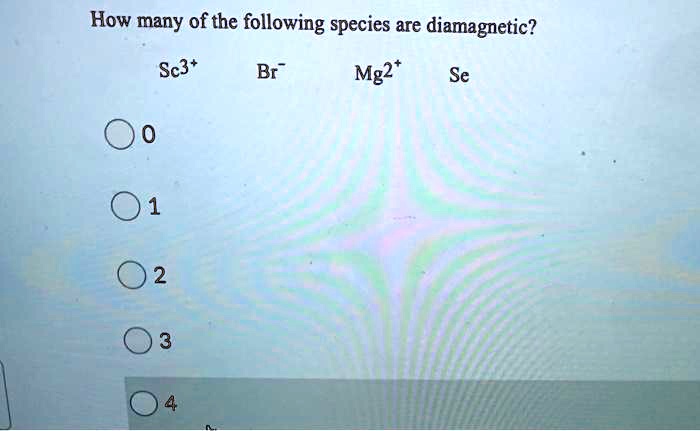 Source: numerade.com
Source: numerade.com
Diamagnetic species are those species which have paired electrons in their molecular orbitals. Cn+ о 1 and 3 only o 1 only o 2 and 3 only o 1, 2, and 3. To know the magnetic character of molecules we can use mo diagram.

Keep in mind that if we have only one atom, then we are talking about atomic orbitals and its subshells, shells etc. The electronic configuration of no will be (ii) no 2. Since the 4d and 5s subshells are entirely filled, there are no unpaired electrons and sn2+ by definition is diamagnetic.
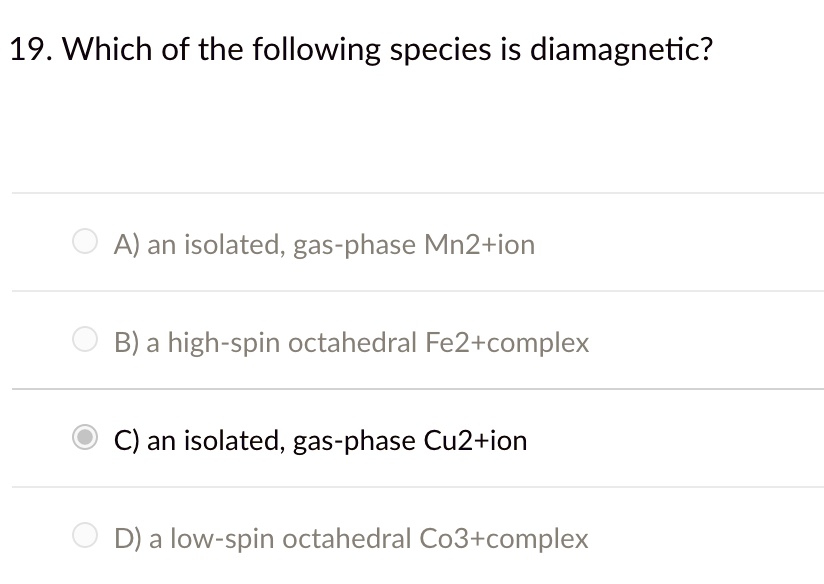 Source: itprospt.com
Source: itprospt.com
Generally, all materials have the diamagnetic properties, making a weak contribution to the magnetic behaviour of the material when subjected to an external magnetic field. We review their content and use your feedback to keep the quality high. What is the magnetic property of c2?
 Source: toppr.com
Source: toppr.com
In this problem, i can write the expression of as to add sigma one h two, seeing my start one is zero. But, in materials that show other magnetic properties such as paramagnetism and ferromagnetism, the effect of diamagnetism is negligible. Orbitals sigma_(1s)^2 , sigma_(1s)^(**2),sigma_(2s)^(**2),sigma_(2s)^(**2),sigma_(2pz)^(2),pi_(2px)^2= pi_(2py)^2, pi_(2px)^(**2)=pi_(2py)^(**2).
 Source: chegg.com
Source: chegg.com
← prev questionnext question →. So according to the option, option b eat correct answer ups and be it correct answer. Similarly if the species contain unpaired electron it is said to be paramagnetic.
 Source: toppr.com
Source: toppr.com
If the electronic configuration of a molecule has only paired or spin paired electrons, then that molecule is said to be diamagnetic. Co + molecule is therefore paramagnetic. Number of valance electrons in co +=4+6−1=9.
Also Read :





