An important measurement that can be made to determine if a solution is acidic is the ph. The p h of a most acidic solution is 1.
Which Solution Is The Most Acidic. The smallest numerical value of ph among them is the most acidic. But how am i supposed to According to the classical definition, a superacid is an acid with an acidity greater than that of 100% pure sulfuric acid, which has a hammett acidity function (h 0) of −12.according to the modern definition, a superacid is a medium in which the chemical potential of the proton is higher than in pure sulfuric acid. Neutral solutions have p h 7.
 Acids And Bases Chapter 7 Web-Site: - Ppt Download From slideplayer.com
Acids And Bases Chapter 7 Web-Site: - Ppt Download From slideplayer.com
Related Post Acids And Bases Chapter 7 Web-Site: - Ppt Download :
Since, ph defines the acidity of a solutions and shows the presence of in it. This type of acids termed as. An important measurement that can be made to determine if a solution is acidic is the ph. Which of the following solutions is acidic?
The ph is a measure of the.
A ph scale measure can vary from 0 to 14, where 0 is the most acidic and 14 is the most basic a substance can be. Acidic and basic are two extremes that describe a chemical property chemicals. Assuming equal concentrations , rank these solutions by ph. This type of acids termed as superacids. Commercially available superacids include trifluoromethanesulfonic. Vinegar�s acidic nature makes it an excellent choice for removing hard water stains from glass and rust stains from sinks.
 Source: numerade.com
Source: numerade.com
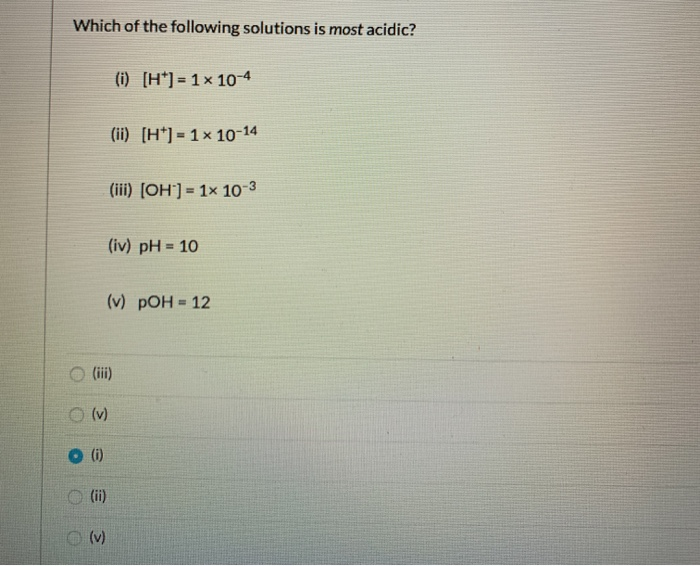
We have to determine which of the following values describes the most acidic solution. Which of the following solutions is acidic? Since, ph defines the acidity of a solutions and shows the presence of in it.
![Solved:given Three Solutions Solution X Has A Ph = 6.50 Solution Y Has A Poh = 10.30 Solution Z Has A [Oh] =42X 10-9 M Which Solution Is The Most Acidic? 0 Solved:given Three Solutions Solution X Has A Ph = 6.50 Solution Y Has A Poh = 10.30 Solution Z Has A [Oh] =42X 10-9 M Which Solution Is The Most Acidic? 0](https://cdn.numerade.com/ask_images/7154ed230ff64aad9c4eba064f66ee76.jpg) Source: numerade.com
Source: numerade.com
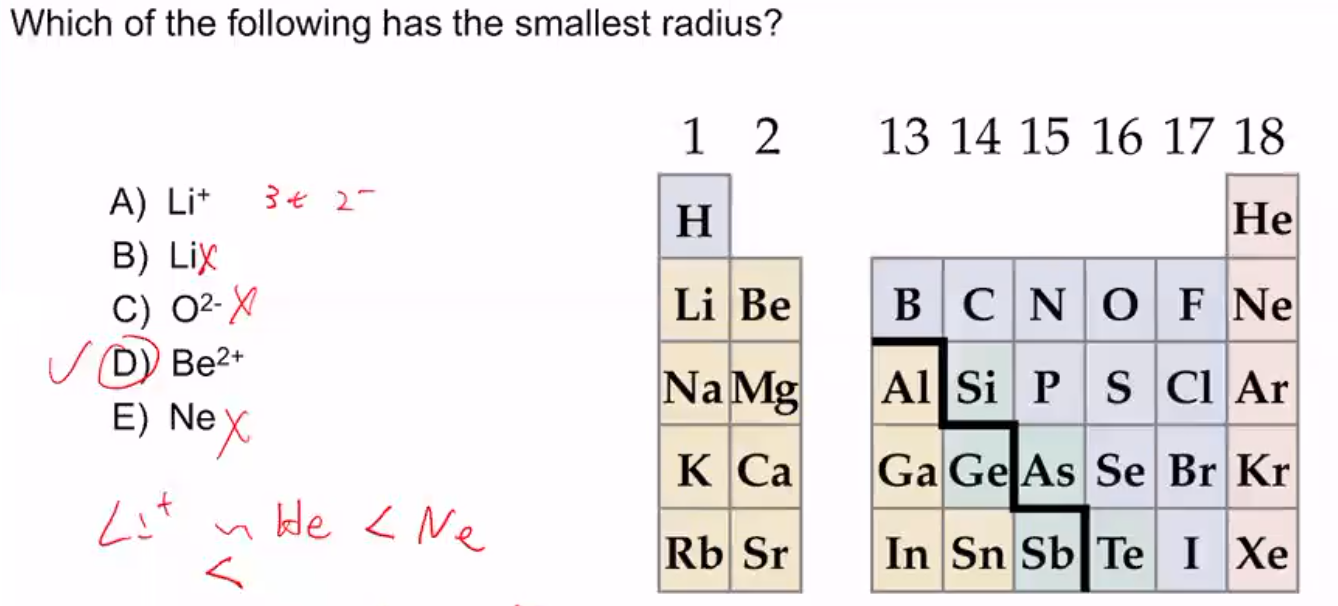
Acidic value ranges from 1 to 7 and towards 1 is more acidic,so solution having ph=3.2 is more acidic. Now in their corresponding compounds, hydrogen will be more strongly bonded with o 2 − and least strongly with t e 2 − because of larger size of t e 2 −, so it will be very easy to remove h+ ions from the other specific presents.so h 2 t e is most acidic hydride. Use that to convert poh 10.1 and 12.8 to ph and compare all for values.
 Source: chegg.com
Source: chegg.com
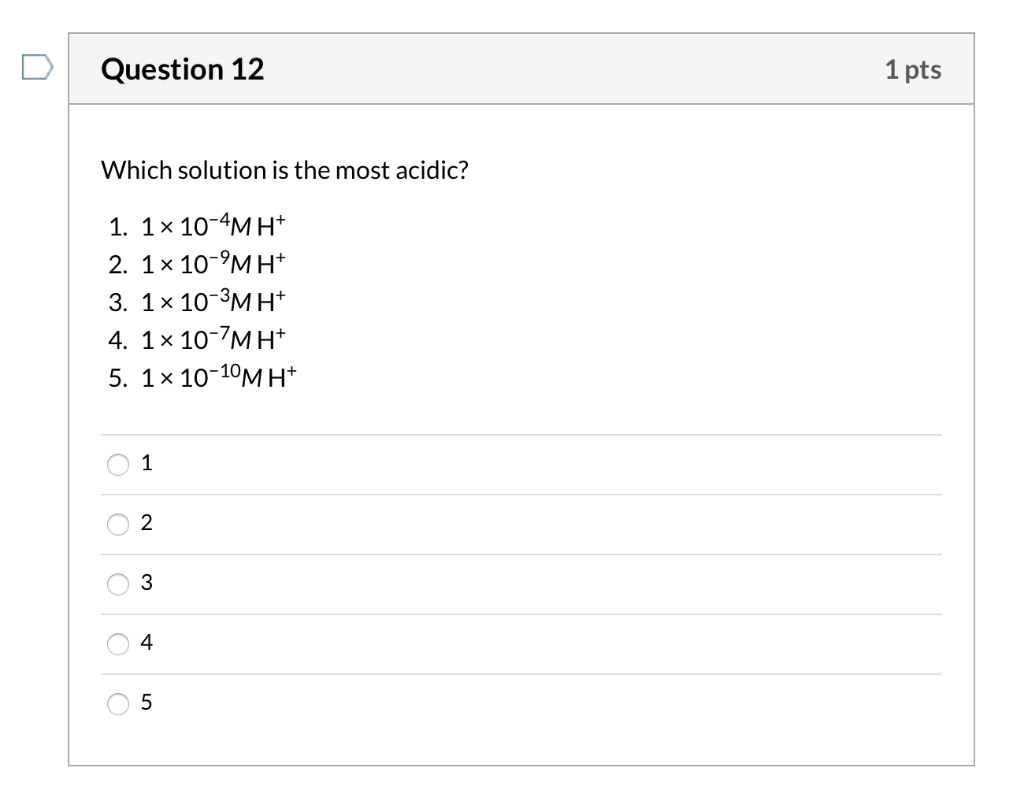
But how am i supposed to Asked jul 9, 2018 in chemistry by anukriti bharti (38.2k points) you have four solutions a, b, c and d. Of values describes the most acidic solution.
 Source: toppr.com
Source: toppr.com
Commercially available superacids include trifluoromethanesulfonic. The most basic solution has been solution b, and the least acidic solution has been solution a, as it has been more basic thus least acidic. Acidic and basic are two extremes that describe a chemical property chemicals.
 Source: chegg.com
Source: chegg.com
Acetic acid is much weaker than citric acid, with a pka of 4.74. Solution for which solution is most acidic (that is, has the lowest ph)?(a) 1.0 m hcl(b) 2.0 m hf(c) a solution that is 1.0 m in hf and 1.0 m in hclo The smallest numerical value of ph among them is the most acidic.

Of values describes the most acidic solution. This type of acids termed as superacids. Neutral solutions have p h 7.
 Source: slideplayer.com
Source: slideplayer.com
The p h of a most acidic solution is 1. This type of acids termed as. According to the classical definition, a superacid is an acid with an acidity greater than that of 100% pure sulfuric acid, which has a hammett acidity function (h 0) of −12.according to the modern definition, a superacid is a medium in which the chemical potential of the proton is higher than in pure sulfuric acid.
![Solved Which Of These Solutions Is The Most Acidic? O [Oh�] | Chegg.com Solved Which Of These Solutions Is The Most Acidic? O [Oh�] | Chegg.com](https://media.cheggcdn.com/study/bd4/bd47f438-6c96-457c-b864-c602b104b99e/image)
Asked jul 9, 2018 in chemistry by anukriti bharti (38.2k points) you have four solutions a, b, c and d. This type of acids termed as superacids. The most acidic solution has been solution c.

Vinegar is a solution of acetic acid and water. Which of the following solutions is acidic? As you climb the ph scale, the next most acidic items are lemon juice and vinegar, each with a ph of around 2.
 Source: chegg.com
Source: chegg.com
The ph scale measures how acidic or. In chemistry, ph (/ p iː ˈ eɪ tʃ /, historically denoting potential of hydrogen (or power of hydrogen) is a scale used to specify the acidity or basicity of an aqueous solution.acidic solutions (solutions with higher concentrations of h + ions) are measured to have lower ph values than basic or alkaline solutions. Commercially available superacids include trifluoromethanesulfonic.
 Source: slidetodoc.com
Source: slidetodoc.com
Acidic and basic are two extremes that describe a chemical property chemicals. Vinegar�s acidic nature makes it an excellent choice for removing hard water stains from glass and rust stains from sinks. Commercially available superacids include trifluoromethanesulfonic.
 Source: quora.com
Source: quora.com
The ph is a measure of the. Solution for which solution below is the most acidic? Neutral solutions have p h 7.

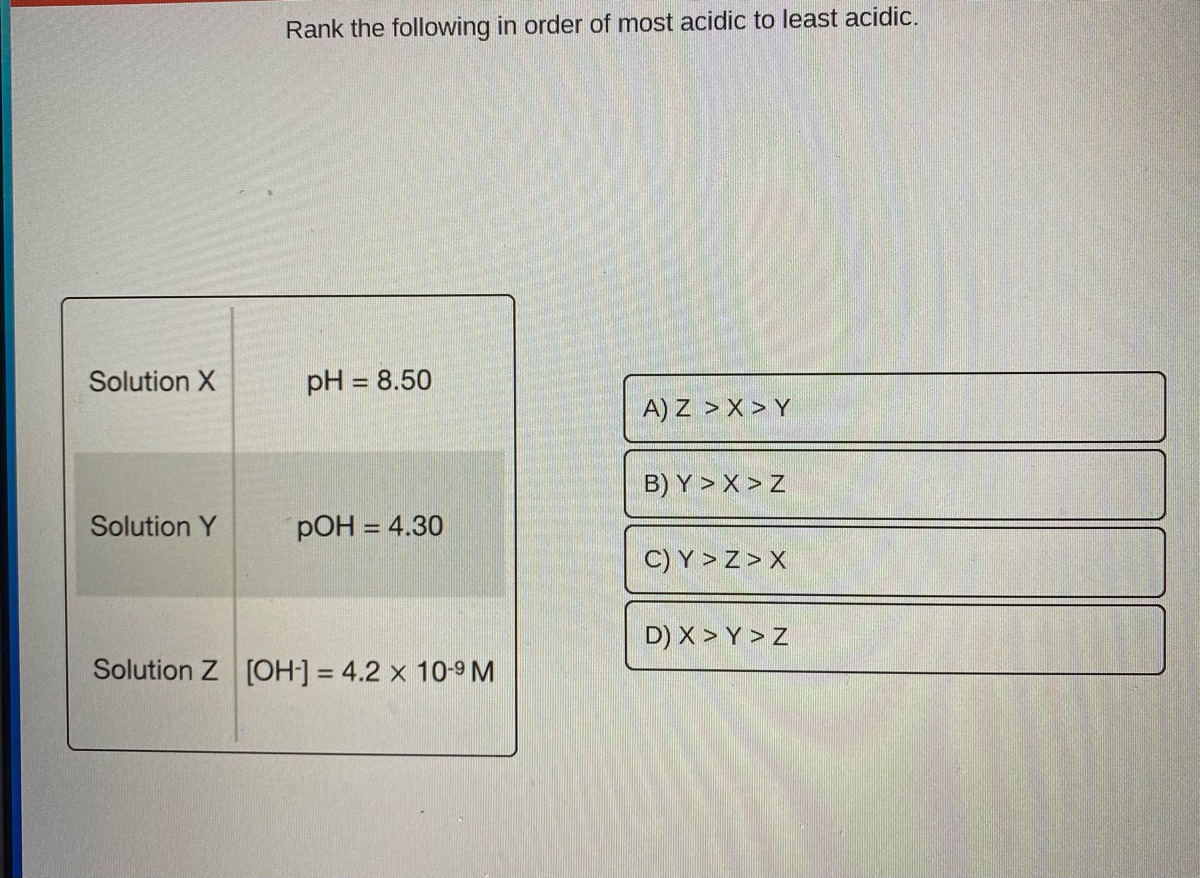
Use that to convert poh 10.1 and 12.8 to ph and compare all for values. Ph of solution c has been: We have to determine which of the following values describes the most acidic solution.
 Source: oneclass.com
Source: oneclass.com
Acidic and basic are two extremes that describe a chemical property chemicals. Solution for which solution is most acidic (that is, has the lowest ph)?(a) 1.0 m hcl(b) 2.0 m hf(c) a solution that is 1.0 m in hf and 1.0 m in hclo The ph scale is logarithmic and inversely indicates the.
 Source: youtube.com
Source: youtube.com
The smallest numerical value of ph among them is the most acidic. In chemistry, ph (/ p iː ˈ eɪ tʃ /, historically denoting potential of hydrogen (or power of hydrogen) is a scale used to specify the acidity or basicity of an aqueous solution.acidic solutions (solutions with higher concentrations of h + ions) are measured to have lower ph values than basic or alkaline solutions. 0.1m solution of which of the following substances is most acidic.

Lower is the p h value, higher is the acidic strength. As you climb the ph scale, the next most acidic items are lemon juice and vinegar, each with a ph of around 2. Vinegar is a solution of acetic acid and water.
 Source: oneclass.com
Source: oneclass.com
We have to determine which of the following values describes the most acidic solution. We have to determine which of the following values describes the most acidic solution. Since, ph defines the acidity of a solutions and shows the presence of in it.
 Source: bartleby.com
Source: bartleby.com
An important measurement that can be made to determine if a solution is acidic is the ph. An important measurement that can be made to determine if a solution is acidic is the ph. Of values describes the most acidic solution.
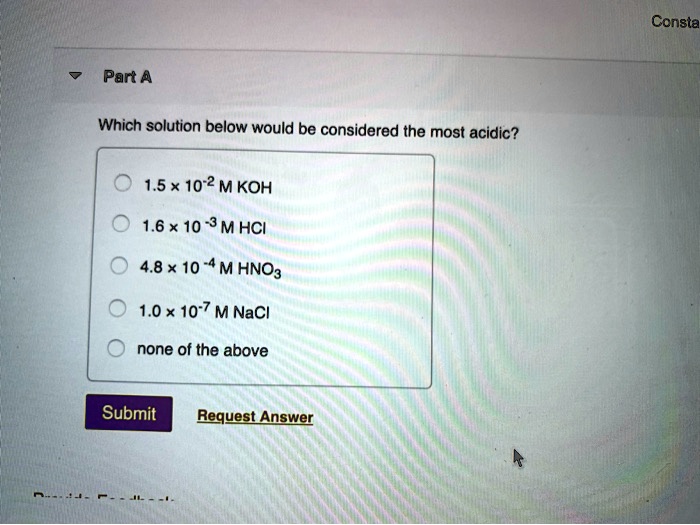 Source: numerade.com
Source: numerade.com
But how am i supposed to Its range varies from 1 to 14 and with increase in number the acidity of the. 0.1m solution of which of the following substances is most acidic.
 Source: study.com
Source: study.com
The ph scale is logarithmic and inversely indicates the. Some vinegar can be nearly as acidic as lemon juice, with a ph of around 2.4, while other types are much more basic, with a ph of over 3. Solution for which solution is most acidic (that is, has the lowest ph)?(a) 1.0 m hcl(b) 2.0 m hf(c) a solution that is 1.0 m in hf and 1.0 m in hclo
Also Read :