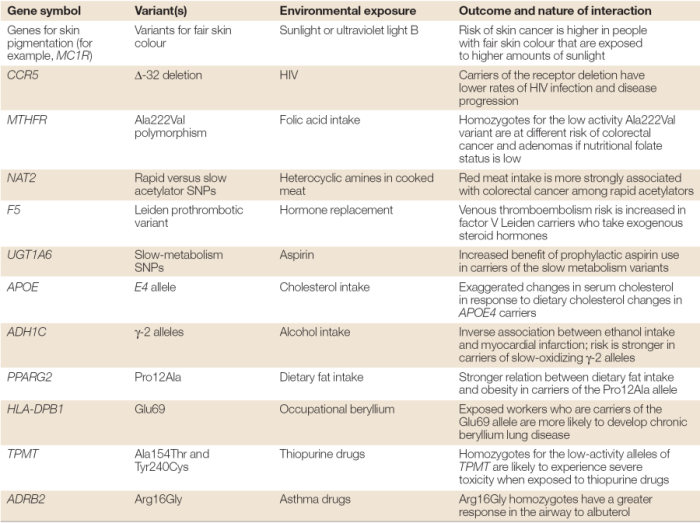
300 times higher than the least conductive metal (pu at 6.74); Conductivity of heat and electricity.
Which Property Best Accounts For The Conductivity Of Metals. They are good electrical conductors because the electrons flow freely in. It is one of the cheapest material used in cutting tools for different machining processes. The delocalised electrons are free to move in the solid lattice. Metals are insoluble in water or any other solvents.their oxides are soluble.

Related Post Why Do Metals Conduct Electricity? – Materials Science & Engineering :
This high thermal conductivity is used by. And nearly 4,000 times that of water (0.58) and 100,000 times that of air (0.0224). It means they can hold heavy weights. Properties of metals the structure and bonding of metals explains their properties :
Conductivity of heat and electricity.
The mobility of the electron fluid in metals is practically unaffected by temperature, but metals do suffer a slight conductivity decrease (opposite to ionic solutions) as the temperature rises; Its thermal conductivity (2,200 w/m•k) is five times greater than the most conductive metal (ag at 429); Metals have shiny surfaces.hence metals like gold, silver, platinum, copper have heavy use for decorative jewelry. I will give a brief account (and a good reference) below. This happens because the more vigorous thermal motions of the kernel ions disrupts the uniform lattice structure that is required for free motion of the electrons within the. Metallic bonding accounts for many physical properties of metals, such as strength, ductility, thermal and electrical resistivity and conductivity, opacity, and luster.

Metals conduct electricity at all. Diamond is the best natural conductor of heat; In fact, for crystalline, nonmetallic solids such as diamond, k ph can be quite large, exceeding values of k associated with good conductors, such as aluminum.

Some metals have properties that are not typical. The metal is found in the earth’s crust in the pure, free elemental form (“native silver”), as an alloy with gold and other metals, and in minerals such as argentite and chlorargyrite. The number of conduction electrons is constant, depending on neither temperature nor impurities.
 Source: thermtest.com
Source: thermtest.com
Their powders are added into making metallic paints. 37 rows electrical conductivity in metals is a result of the movement of. The aluminum atom has three valence electrons in a partially filled outer shell.
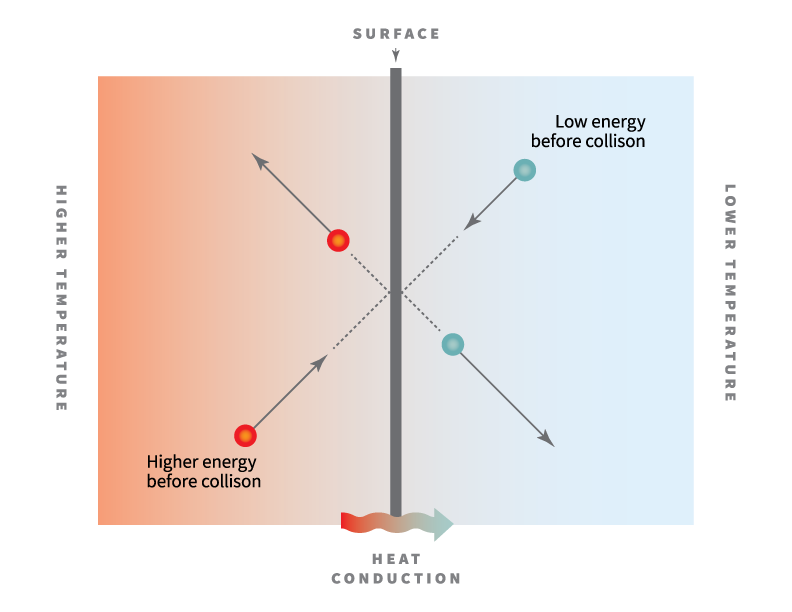
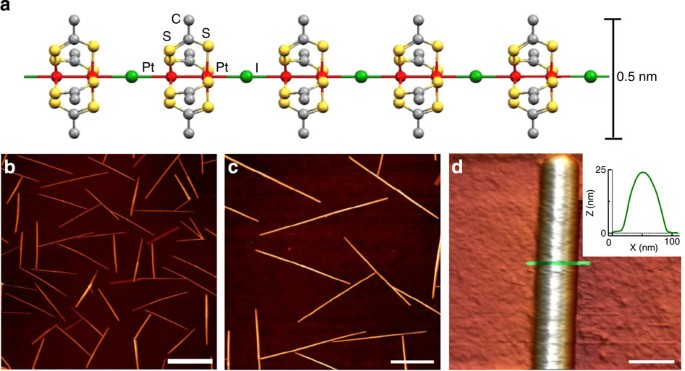 Source: nature.com
Source: nature.com
The free electrons of the metals can freely move throughout the solid and transfer the thermal energy at a rate very high as compared to insulators. Phonons play a major role in many of the physical properties of condensed matter, like thermal conductivity and electrical conductivity. Silver is a soft, white, lustrous transition metal, it exhibits the highest electrical conductivity, thermal conductivity, and reflectivity of any metal.
 Source: sciencedirect.com
Source: sciencedirect.com
It means they can hold heavy weights. It even feels cold to the touch. Metals conduct electricity at all.

Silver is a soft, white, lustrous transition metal, it exhibits the highest electrical conductivity, thermal conductivity, and reflectivity of any metal. They are electrical conductors because their delocalised electrons. 37 rows electrical conductivity in metals is a result of the movement of.

It means they can hold heavy weights. They are good electrical conductors because the electrons flow freely in. Since both electrical as well as thermal.
 Source: azom.com
Source: azom.com
Metallic bonding accounts for many physical properties of metals, such as strength, malleability, ductility, thermal and electrical conductivity, opacity, and luster. Metallic bonding accounts for many physical properties of metals, such as strength, malleability, ductility, thermal and electrical conductivity, opacity, and luster. Metals have useful properties including strength, ductility, high melting points, thermal and electrical conductivity, and toughness.

From the periodic table, it can be seen that a large number of the elements are classified as being a metal. And nearly 4,000 times that of water (0.58) and 100,000 times that of air (0.0224). It is due to this that the metals posses high thermal conductivity.
 Source: studylib.net
Source: studylib.net
37 rows electrical conductivity in metals is a result of the movement of. List of different cutting tool materials available for different machining processes: The electron sea model explains many of the physical properties of metals.
 Source: slideplayer.com
Source: slideplayer.com
Metallic bonding accounts for many physical properties of metals, such as strength, ductility, thermal and electrical resistivity and conductivity, opacity, and luster. The electron sea model explains many of the physical properties of metals. In fact, for crystalline, nonmetallic solids such as diamond, k ph can be quite large, exceeding values of k associated with good conductors, such as aluminum.
 Source: coursehero.com
Source: coursehero.com
Metals have shiny surfaces.hence metals like gold, silver, platinum, copper have heavy use for decorative jewelry. It means when we strike them, they make a ringing sound. And nearly 4,000 times that of water (0.58) and 100,000 times that of air (0.0224).
 Source: sciencedirect.com
Source: sciencedirect.com
The delocalised electrons are free to move in the solid lattice. This high thermal conductivity is used by. Since both electrical as well as thermal.

Metallic bonding accounts for many physical properties of metals, such as strength, ductility, thermal and electrical resistivity and conductivity, opacity, and luster. Metals have good tensile strength so they can be molded into different shapes. Gold is used as a contact metal in the electronics industry as it is a good conductor of both electricity and heat.
 Source: thoughtco.com
Source: thoughtco.com
It means they cannot be cut easily. Metals are often described as positive ions in a sea of electrons. Metals are insoluble in water or any other solvents.their oxides are soluble.
 Source: education.com
Source: education.com
Metals are insoluble in water or any other solvents.their oxides are soluble. Diamond is the best natural conductor of heat; This material is also easy to make.
 Source: en.wikipedia.org
Source: en.wikipedia.org
From the periodic table, it can be seen that a large number of the elements are classified as being a metal. Diamond is the best natural conductor of heat; Metals have a high density of conduction electrons.
 Source: thoughtco.com
Source: thoughtco.com
Silver is a soft, white, lustrous transition metal, it exhibits the highest electrical conductivity, thermal conductivity, and reflectivity of any metal. Metals have useful properties including strength, ductility, high melting points, thermal and electrical conductivity, and toughness. The mobility of the electron fluid in metals is practically unaffected by temperature, but metals do suffer a slight conductivity decrease (opposite to ionic solutions) as the temperature rises;
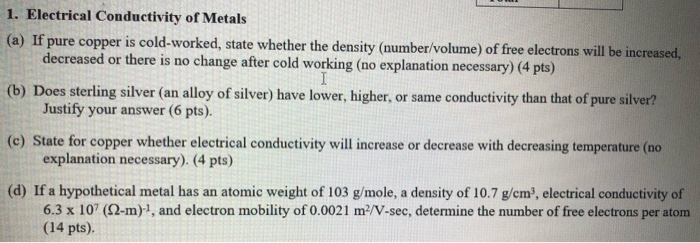 Source: chegg.com
Source: chegg.com
Their powders are added into making metallic paints. They are good electrical conductors because the electrons flow freely in. They are electrical conductors because their delocalised electrons.

Gold is used as a contact metal in the electronics industry as it is a good conductor of both electricity and heat. The number of conduction electrons is constant, depending on neither temperature nor impurities. The free electrons of the metals can freely move throughout the solid and transfer the thermal energy at a rate very high as compared to insulators.
Also Read :