Asked jun 23, 2017 in chemistry by debiwebi. The kinetic molecular theory of gases has the following postulates:
Which Postulate Of The Kinetic Molecular Theory. Gases are composed of very tiny particles (molecules). I would say that the main postulate for this theory is statistical mechanics. The kinetic molecular theory describes the properties of molecules in terms of motion (kinetic energy) and of temperature. Lower kinetic energy reduces successful collisions and thus reduces reactivity and the number of interactions.
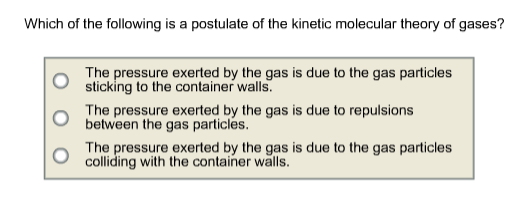 Solved Which Of The Following Is A Postulate Of The Kinetic | Chegg.com From chegg.com
Solved Which Of The Following Is A Postulate Of The Kinetic | Chegg.com From chegg.com
Related Post Solved Which Of The Following Is A Postulate Of The Kinetic | Chegg.com :
The volume occupied by the individual particles that compose a gas is very small. Gases are composed of very tiny particles (molecules). The kinetic molecular theory of matter states that: It states that the molecules present in the gases are very small in comparison to the distance between the molecules.
All particles have energy, but the energy varies depending on the temperature the sample of matter is in.
The theory assumes that gases consist of widely separated molecules of negligible volume that are in constant motion, colliding elastically with one another and the walls of their container with average velocities determined by their absolute. Pressure does not have any effect on the kinetic energy of the molecule. All particles have energy, but the energy varies depending on the temperature the sample of matter is in. Which of the following is included as a postulate in the kinetic molecular theory of an ideal gas? The space occupied by the individuals gas molecules is negligible compared to the total volume of the gas. 3 postulates of the kinetic molecular theory:
 Source: chegg.com
Source: chegg.com
The kinetic molecular theory of matter states that: This in turn determines whether the substance exists in the solid, liquid, or gaseous state. 1) volume of real gases are negligible and small.
 Source: brainly.in
Source: brainly.in
This fact explains the compressibility of gases. Matter is made up of particles that are constantly moving.liquid molecules have more kinetic energy than those. Which of the following cannot be used to state a postulate?
 Source: slideplayer.com
Source: slideplayer.com
- the average kinetic energy of gases are directly proportional to the absolute temperature in. Which of the following is a postulate of the kinetic molecular theory of gases?a. At lower temperatures the molecules have reduced kinetic energy.
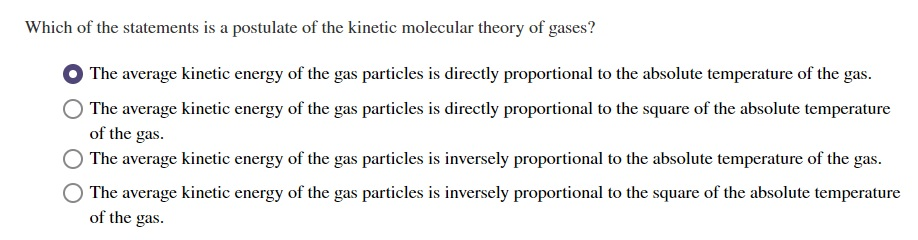 Source: chegg.com
Source: chegg.com
The kinetic energy of the molecule directly proportional to the temperature. Matter is made up of particles that are constantly moving.liquid molecules have more kinetic energy than those. The key to this explanation is the last postulate of the kinetic theory, which assumes that the temperature of a system is proportional to the average kinetic energy of its particles and nothing else.
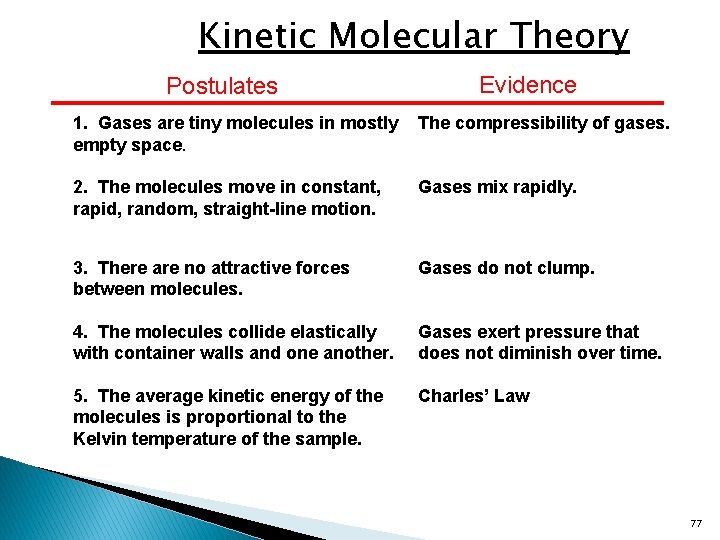 Source: slidetodoc.com
Source: slidetodoc.com
So at low temperatures, the postulate that breaks down is that the forces between the gas particles are not significant. The kinetic molecular theory describes the properties of molecules in terms of motion (kinetic energy) and of temperature. And so the reason that this happens is that low temperatures the molecules are not moving as fast, and so when they collide, they have more time to interact.
 Source: slideserve.com
Source: slideserve.com
Kinetic molecular theory can be used to explain both charles’ and boyle’s laws. Um and there are pretty much basically two of those. Lower kinetic energy reduces successful collisions and thus reduces reactivity and the number of interactions.

Which postulate or theorem proves that these two triangles are congruent? Matter is made up of particles that are constantly moving.liquid molecules have more kinetic energy than those. Pressure does not have any effect on the kinetic energy of the molecule.
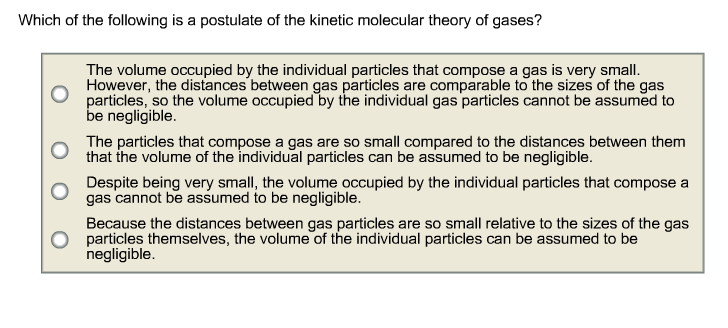 Source: chegg.com
Source: chegg.com
The theory assumes that gases consist of widely separated molecules of negligible volume that are in constant motion, colliding elastically with one another and the walls of their container with average velocities determined by their absolute. The kinetic molecular theory of gases has the following postulates: The kinetic molecular theory is a simple but very effective model that effectively explains ideal gas behavior.
 Source: unacademy.com
Source: unacademy.com
One of them is the part that says that there are no attractions between molecules. Which of the following cannot be used to state a postulate? The kinetic molecular theory of matter states that:
 Source: slideplayer.com
Source: slideplayer.com
The theory assumes that gases consist of widely separated molecules of negligible volume that are in constant motion, colliding elastically with one another and the walls of their container with average velocities determined by their absolute. The particles are in constant random motion, colliding with the walls of the container. The kinetic molecular theory is a microscopic model that helps understand what happens to gas particles at the molecular or atomic level when conditions such as.
 Source: slideserve.com
Source: slideserve.com
The kinetic molecular theory can be used to explain the results graham obtained when he studied the diffusion and effusion of gases. Asked jun 23, 2017 in chemistry by debiwebi. Without this we cannot derive any �real� predictions.
 Source: quora.com
Source: quora.com
The space occupied by the individuals gas molecules is negligible compared to the total volume of the gas. Matter is made up of particles that are constantly moving.liquid molecules have more kinetic energy than those. This fact explains the compressibility of gases.
 Source: slideplayer.com
Source: slideplayer.com
This fact explains the compressibility of gases. Matter is made up of particles that are constantly moving.liquid molecules have more kinetic energy than those. Kinetic molecular theory can be used to explain both charles� and boyle�s laws.
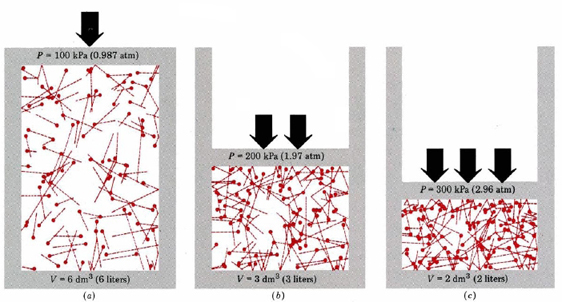 Source: chem.libretexts.org
Source: chem.libretexts.org
The actual volume of these molecules is so small as to be negligible compared with the total volume of the gas sample. One of them is the part that says that there are no attractions between molecules. Matter is made up of particles that are constantly moving.
 Source: brainly.in
Source: brainly.in
- the average kinetic energy of gases are directly proportional to the absolute temperature in. Kinetic molecular theory can be used to explain both charles’ and boyle’s laws. The theory is most often applied to gases but is helpful in explaining molecular behavior in all states of matter.
 Source: co.pinterest.com
Source: co.pinterest.com
Which of the following is a postulate of the kinetic molecular theory of gases? The kinetic molecular theory of matter states that: As applied to gases, the kinetic molecular theory has the following postulates:
 Source: youtube.com
Source: youtube.com
So this question asks what postulate of the kinetic molecular theory breaks down under low temperatures. The kinetic molecular theory and graham�s laws the kinetic molecular theory can be used to explain the results graham obtained when he studied the diffusion and effusion of gases. 1) volume of real gases are negligible and small.
 Source: unacademy.com
Source: unacademy.com
The kinetic molecular theory of matter states that: 3 postulates of the kinetic molecular theory: This fact explains the compressibility of gases.
 Source: slideplayer.com
Source: slideplayer.com
I would say that the main postulate for this theory is statistical mechanics. The kinetic molecular theory can be used to explain the results graham obtained when he studied the diffusion and effusion of gases. So at low temperatures, the postulate that breaks down is that the forces between the gas particles are not significant.
 Source: slideserve.com
Source: slideserve.com
The molecules are in a continuous random motion, occupy space, and interact with each other through constant collision. This in turn determines whether the substance exists in the solid, liquid, or gaseous state. The kinetic molecular theory is a simple but very effective model that effectively explains ideal gas behavior.
Also Read :




