This organocatalyst consists of carbon, hydrogen, sulfur and other nonmetal elements found in organic compounds. Serine proteases use four of the major catalytic mechanism during the reaction cycle:
Which Organic Molecule Serves As A Catalyst. Olefins are among nature’s most abundant chemical compounds and commonly obtained from. Protonation of the oxygen atom increases the polarity of the carbonyl bond. The product of this reaction then loses an h + ion to form. A macromolecules serving as a catalyst, a chemical agent that increases the rate of a reaction without being consumed by the reaction.
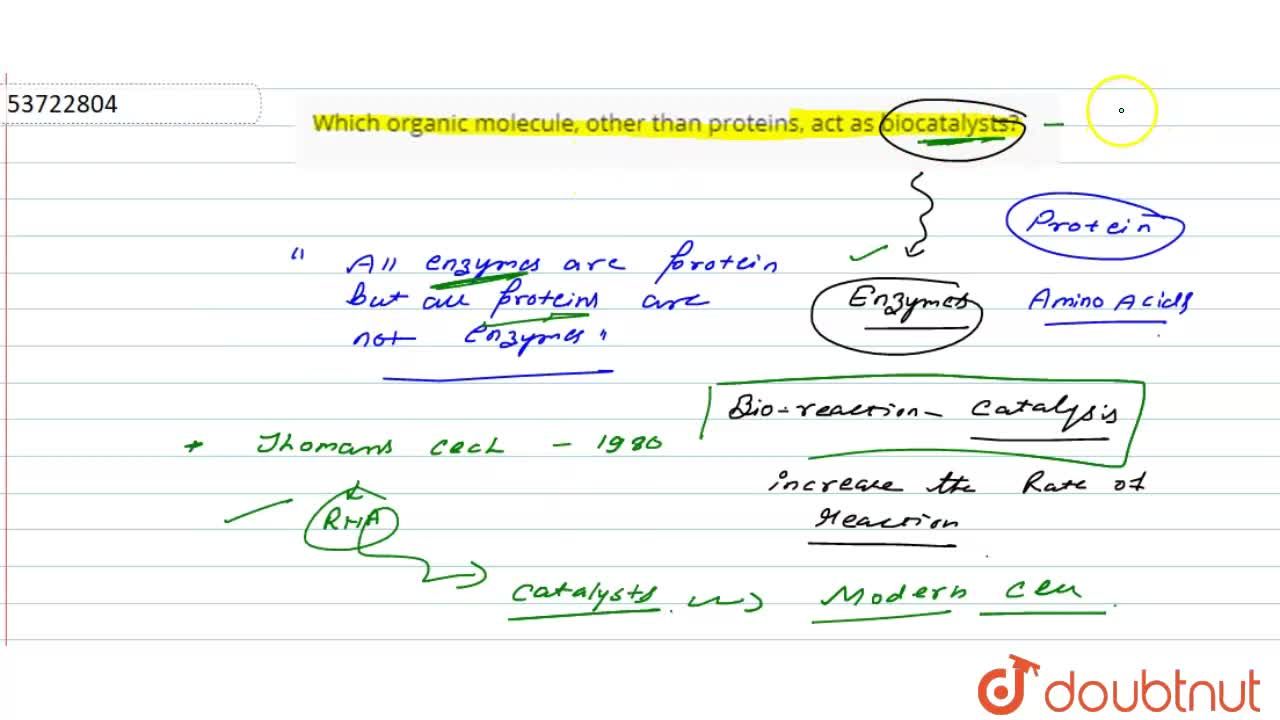 Which Organic Molecule, Other Than Proteins, Act As Biocatalysts? From doubtnut.com
Which Organic Molecule, Other Than Proteins, Act As Biocatalysts? From doubtnut.com
Related Post Which Organic Molecule, Other Than Proteins, Act As Biocatalysts? :
A macromolecule, usually a protein, that serves as a biological catalyst, changing the rate of a chemical reaction without being consumed by the reaction. Silverio, d., torker, s., pilyugina, t. And alcohol production by addition to aldehydes and. Enzymes are a specific organic molecule that acts as a catalyst.
This was achieved through asymmetric organocatalysis, a process in which an organic molecule serves as a catalyst that drives a chemical transformation to one desired product.
Which type of substances serves as a catalyst for reactions that break large food molecules into smaller useful molecules? The team specifically studied the role of catalysts in the conversion of inert molecules called olefins. Because of their similarity in composition and description, they are often mistaken as a. This organocatalyst consists of carbon, hydrogen, sulfur and other nonmetal elements found in organic compounds. Study of structure determines their structural formula.study of properties includes physical and chemical properties, and evaluation of chemical reactivity to understand their behavior. The active site of serine proteases contains a catalytic triad of three amino acids:
 Source: onlinelibrary.wiley.com
Source: onlinelibrary.wiley.com
Serine proteases use four of the major catalytic mechanism during the reaction cycle: For alcohols, both primary and secondary alcohols can be oxidized. This increases the rate at which a water molecule can act as a nucleophile toward the positive end of the c=o double bond.
 Source: doubtnut.com
Source: doubtnut.com
Silverio, d., torker, s., pilyugina, t. Dna is genetic material while atp is the energy currency of the cell. Ribozymes are the rna molecules that serve as enzymes.
 Source: sciencedirect.com
Source: sciencedirect.com
The role of the acid catalyst is easy to understand. Dehydration reaction a chemical reaction in which to molecules become covalently bonded to each other with the removal of a water molecule This increases the rate at which a water molecule can act as a nucleophile toward the positive end of the c=o double bond.
 Source: www2.estrellamountain.edu
Source: www2.estrellamountain.edu
This organocatalyst consists of carbon, hydrogen, sulfur and other nonmetal elements found in organic compounds. (e) the activation energies of catalysts a, b, c, and d all result in the same reaction time. Catalytic hydrogenation refers to the addition of hydrogen to an organic molecule in the presence of a catalyst.
 Source: brainly.com
Source: brainly.com
A macromolecule, usually a protein, that serves as a biological catalyst, changing the rate of a chemical reaction without being consumed by the reaction. Simple organic molecules as catalysts for enantioselective synthesis of amines and alcohols. Organic catalysts have a stable framework of carbon atoms, to which more active chemical groups can attach.
 Source: nature.com
Source: nature.com
And alcohol production by addition to aldehydes and. They are usually specific types of proteins, such as pepsin. Which type of substances serves as a catalyst for reactions that break large food molecules into smaller useful molecules?
 Source: doubtnut.com
Source: doubtnut.com
A macromolecules serving as a catalyst, a chemical agent that increases the rate of a reaction without being consumed by the reaction. In a reaction, the spark plug of an engine can best be classified as (a) a catalyst (b) a supplier of activation energy Our studies indicate that small organic molecules as catalysts have the ability to achieve the level of stereoselectivity that is shown by peptide catalysts under ambient conditions.
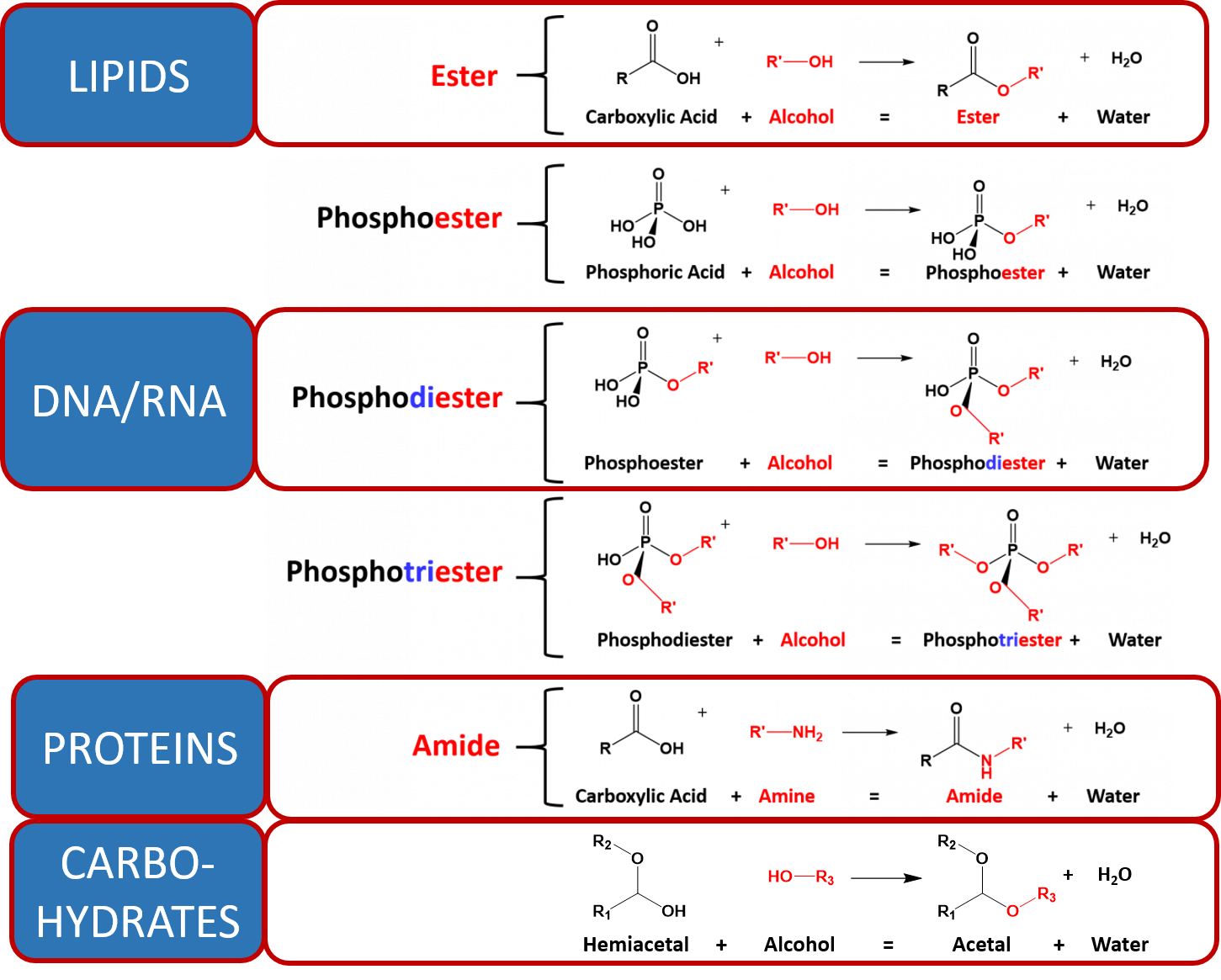 Source: wou.edu
Source: wou.edu
It is unquestionably, vastly important to understand how different noncovalent interactions can be. Since fibrinogen is made of chains of amino acids, it is an example of which type of organic molecule? Ribozymes are the rna molecules that serve as enzymes.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
In organic chemistry, organocatalysis is a form of catalysis in which the rate of a chemical reaction is increased by an organic catalyst. The molecule gaining electrons is being reduced. In organic chemistry, organocatalysis is a form of catalysis in which the rate of a chemical reaction is increased by an organic catalyst.
 Source: www2.estrellamountain.edu
Source: www2.estrellamountain.edu
Enzymes are a specific organic molecule that acts as a catalyst. The product of this reaction then loses an h + ion to form. In organic chemistry, organocatalysis is a form of catalysis in which the rate of a chemical reaction is increased by an organic catalyst.
 Source: researchgate.net
Source: researchgate.net
The oxidizing agent can be a metal or another organic molecule. They are usually specific types of proteins, such as pepsin. The process can involve direct addition of hydrogen to the double bond of an unsaturated molecule;
 Source: onlinelibrary.wiley.com
Source: onlinelibrary.wiley.com
This was achieved through asymmetric organocatalysis, a process in which an organic molecule serves as a catalyst that drives a chemical transformation to one desired product. A macromolecules serving as a catalyst, a chemical agent that increases the rate of a reaction without being consumed by the reaction. Protonation of the oxygen atom increases the polarity of the carbonyl bond.
 Source: advancedsciencenews.com
Source: advancedsciencenews.com
Because of their similarity in composition and description, they are often mistaken as a. Because of their similarity in composition and description, they are often mistaken as a. This was achieved through asymmetric organocatalysis, a process in which an organic molecule serves as a catalyst that drives a chemical transformation to one desired product.
 Source: nature.com
Source: nature.com
Which type of substances serves as a catalyst for reactions that break large food molecules into smaller useful molecules? These often contain common elements such as oxygen, nitrogen, sulphur or phosphorus. The oxidizing agent can be a metal or another organic molecule.
 Source: onlinelibrary.wiley.com
Source: onlinelibrary.wiley.com
(e) the activation energies of catalysts a, b, c, and d all result in the same reaction time. Organic catalysts have a stable framework of carbon atoms, to which more active chemical groups can attach. The study of organic reactions.
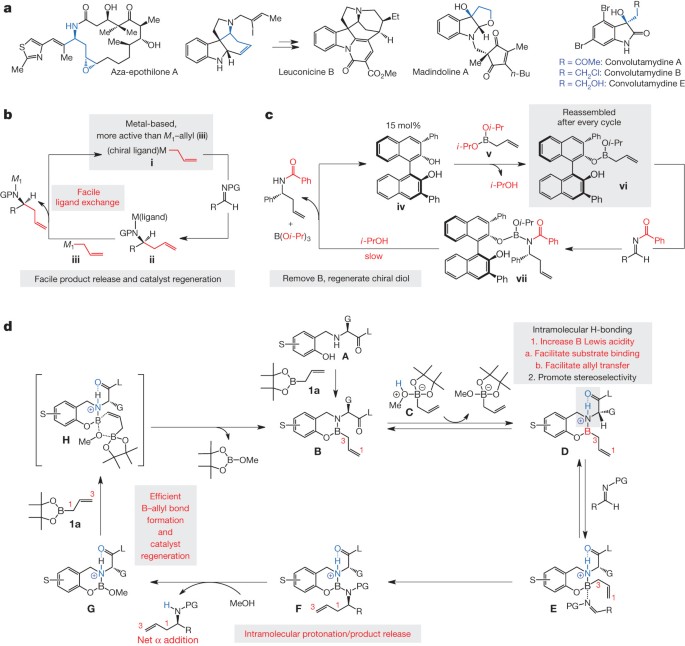 Source: nature.com
Source: nature.com
For alcohols, both primary and secondary alcohols can be oxidized. The active site of serine proteases contains a catalytic triad of three amino acids: The product of this reaction then loses an h + ion to form.
 Source: nature.com
Source: nature.com
The construction of catalysts by supramolecular forces has recently become a powerful tool and the role of noncovalent interactions can assist in designing new tools for the construction of effective and selective catalytic systems. Typically, synthesizing catalysts is tedious experimental work. This organocatalyst consists of carbon, hydrogen, sulfur and other nonmetal elements found in organic compounds.
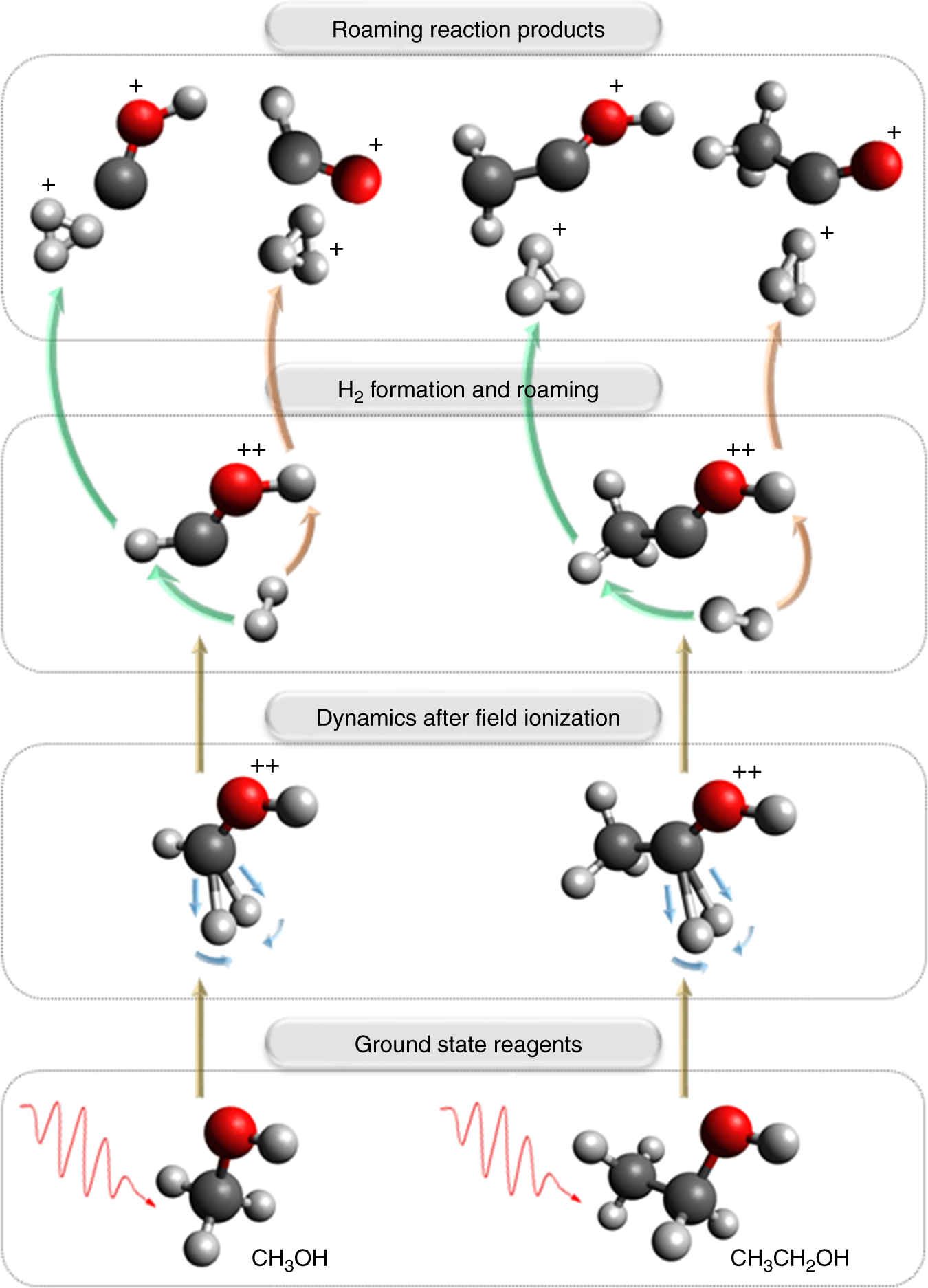 Source: nature.com
Source: nature.com
Typically, synthesizing catalysts is tedious experimental work. Organic catalysts have a stable framework of carbon atoms, to which more active chemical groups can attach. In a reaction, the spark plug of an engine can best be classified as (a) a catalyst (b) a supplier of activation energy
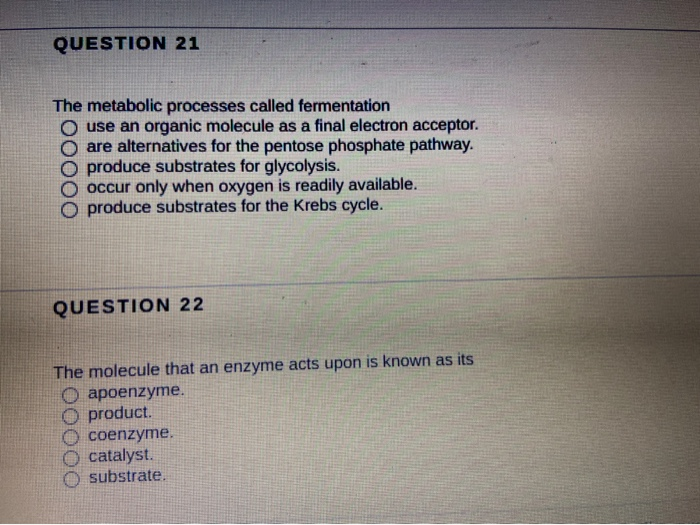 Source: chegg.com
Source: chegg.com
In many oxidation reactions the oxidizing agent is shown above the reaction arrow as [o]. The role of the acid catalyst is easy to understand. Serine proteases use four of the major catalytic mechanism during the reaction cycle:
 Source: onlinelibrary.wiley.com
Source: onlinelibrary.wiley.com
The molecule gaining electrons is being reduced. This increases the rate at which a water molecule can act as a nucleophile toward the positive end of the c=o double bond. Typically, synthesizing catalysts is tedious experimental work.
Also Read :




