C — br → δen = 0.3 → nonpolar bond. C) triatomic molecules in which all bonds are polar must be polar.
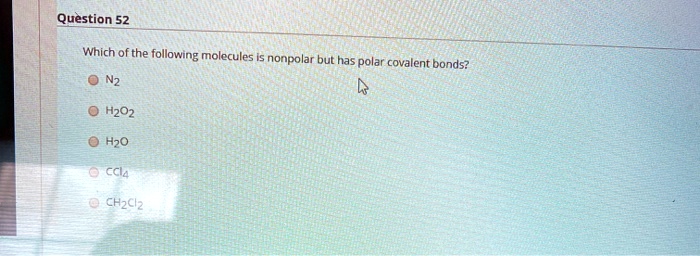
Which One Of The Following Molecules Is Nonpolar. So each of the bond has a typo moment going towards the oxygen. Of c and but all the four c − c 1 bond dipoles cancel each. As a result, they are nonpolar molecules by nature (examples: Which one of the following molecules is nonpolar?

Related Post Solved Which One Of The Following Molecules Is Nonpolar? O | Chegg.com :
The electronegativities of c and 0 are 39) th 2.5 and 3.5, respectively. Molecules in one or more atoms have more than eight electrons (e.g., sf6). A.ethylene has only single bonds. C — br → δen = 0.3 → nonpolar bond.
30.ethylene has the formula c 2 h 4.
P cl5 p c l 5. Which of the following molecules is nonpolar? C — br → δen = 0.3 → nonpolar bond. A) nh3 b) of2 c) ch3cl d) h2o e) becl2. Learn this topic by watching molecular polarity concept videos. The most nonpolar of all the molecules you listed would be nh3.
 Source: oneclass.com
Source: oneclass.com
I think this one is pretty obvious. Which of the following molecules has polar bonds but is a nonpolar molecule? Become a study.com member to unlock this answer!

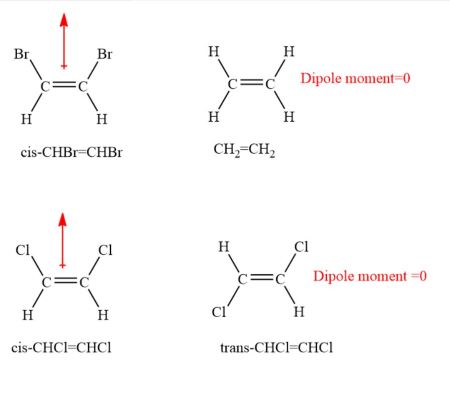
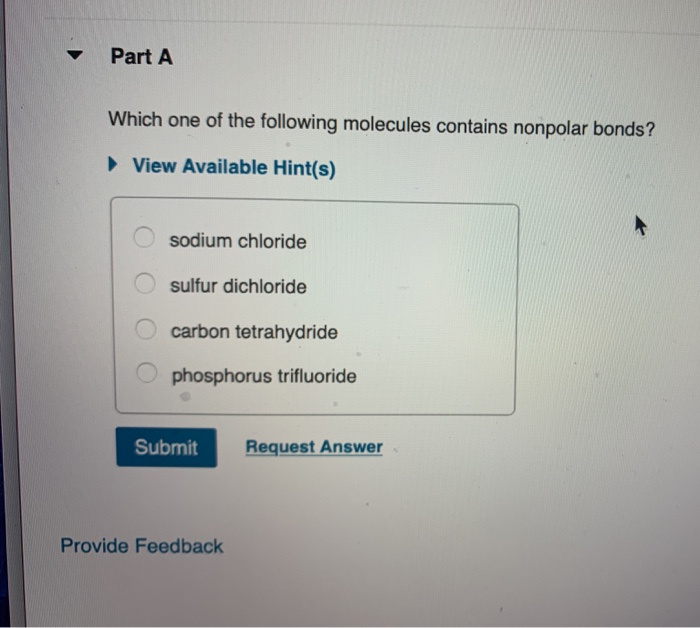
B) all linear triatomic molecules are nonpolar. Part a which one of the following molecules contains nonpolar bonds? The most nonpolar of all the molecules you listed would be nh3.
 Source: numerade.com
Source: numerade.com
A) linear, polar d) bent. An example is the bf3. B) nonpolar bonds, but is a polar molecule.
 Source: docplayer.net
Source: docplayer.net
There are molecules with a polar bond, but the molecular geometry is symmetrical. A) pcl 3 b) ncl 3 c) bf 3 d) hf e) ocl 2 ans: According to v s e p r theory the repulsion between different pair (lone or bond) of electrons obey the order.

Which one of the following molecules is nonpolar? C — br → δen = 0.3 → nonpolar bond. Which one of the following molecules is nonpolar?
 Source: studylib.net
Source: studylib.net
29.which one of the following molecules is nonpolar? C.ethylene has one double bond. D.none of these is correct.
 Source: clutchprep.com
Source: clutchprep.com
All polar molecules have dipole moments. B.ethylene has one triple bond. Of c and but all the four c − c 1 bond dipoles cancel each.
 Source: study.com
Source: study.com
31.what is the name of no 2? Valence shell electron pair repulsion (vsepr) theory. A) becl 2 b) br 2 c) bf 3 d) ibr e) co 2 ans:
Lipids are hydrophobic because the lipid molecules are nonpolar cholesterol, testosterone, progesterone, and the estrogens are nonpolar molecules composed of 4 ring structures. Part a which one of the following molecules contains nonpolar bonds? Be soluble in nonpolar solvents.
 Source: clutchprep.com
Source: clutchprep.com
Contain at least one fatty acid unit. C.ethylene has one double bond. All chemistry practice problems molecular polarity practice problems.
 Source: chegg.com
Source: chegg.com
1)a large nonpolar molecule is likely to be. B) nonpolar bonds, but is a polar molecule. Explain why one of these molecules is polar and the other is nonpolar?

29.which one of the following molecules is nonpolar? Molecules with less than eight electrons; The most nonpolar of all the molecules you listed would be nh3.
 Source: clutchprep.com
Source: clutchprep.com
Which one of the following molecules/ions is nonpolar? A) nonpolar bonds, and is a nonpolar molecule. A.ethylene has only single bonds.
 Source: studylib.net
Source: studylib.net
B.ethylene has one triple bond. Which one of the following molecules is polar? Predict the molecular geometry and polarity of the so 2 molecule.
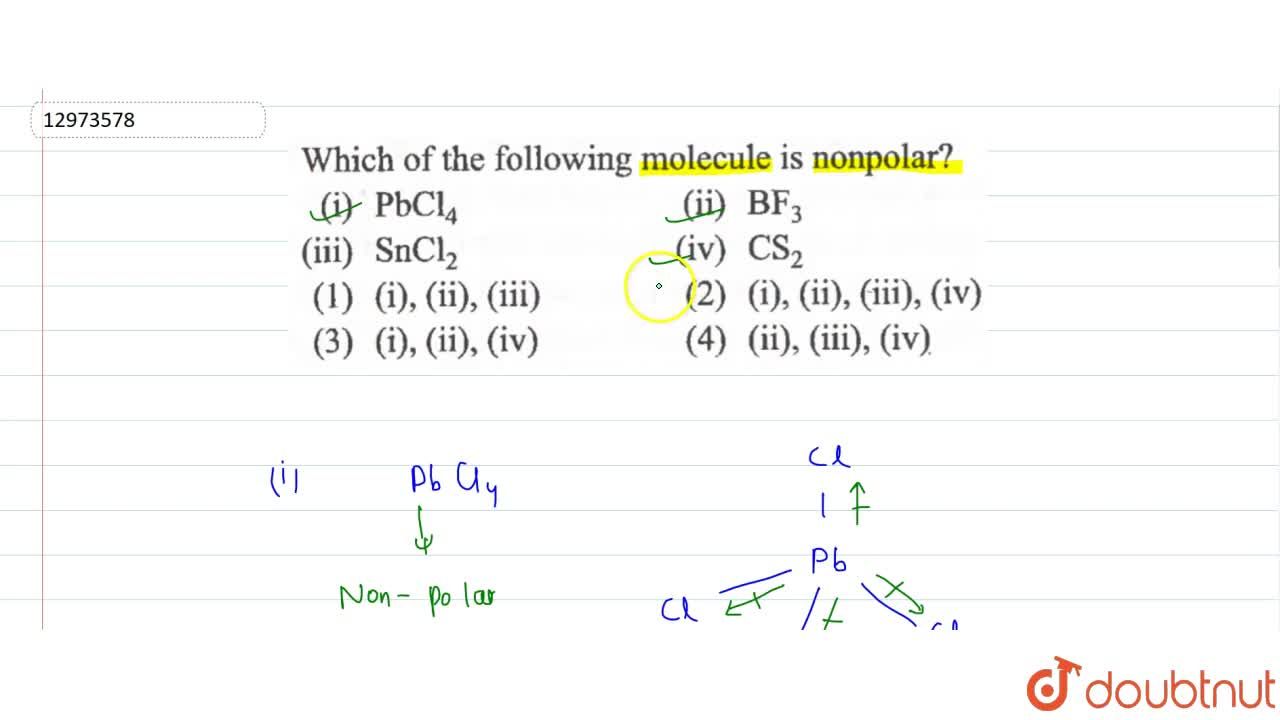 Source: doubtnut.com
Source: doubtnut.com
Be soluble in nonpolar solvents. Which of the following statements is (or are) true? There are molecules with a polar bond, but the molecular geometry is symmetrical.
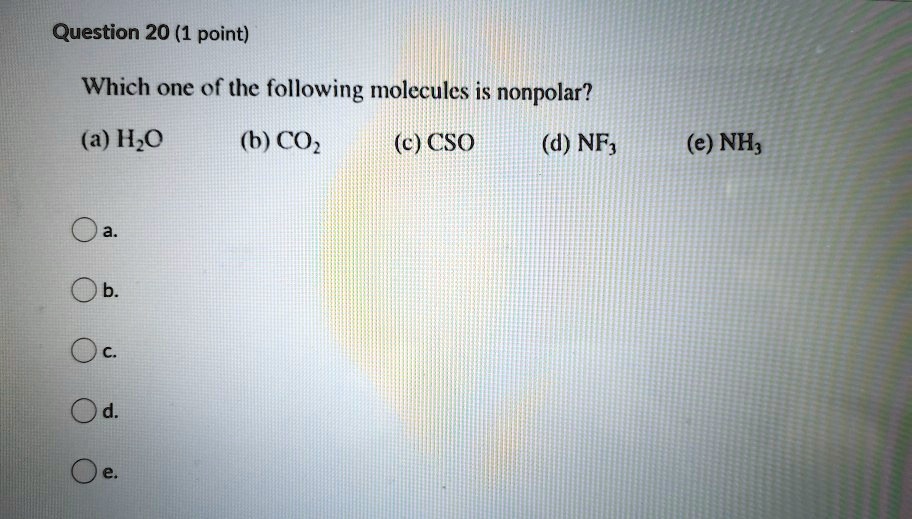 Source: numerade.com
Source: numerade.com
Can you please answer this question: 30.ethylene has the formula c 2 h 4. For example, the cl2 molecule has no polar bonds because the electron charge is identical on both atoms.
 Source: brainly.com
Source: brainly.com
As a result, they are nonpolar molecules by nature (examples: Ccl4, ch2cl2, ch3cl2, ch3cl, chcl3, or sih2cl2? 29.which one of the following molecules is nonpolar?
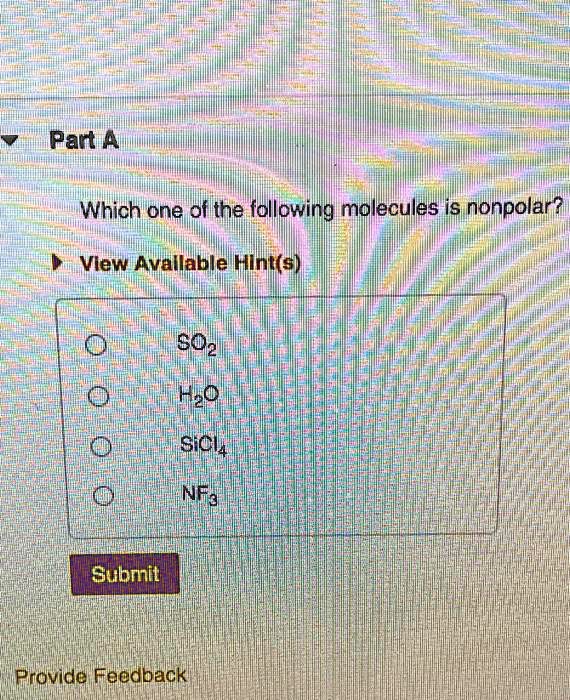 Source: numerade.com
Source: numerade.com
For example, the cl2 molecule has no polar bonds because the electron charge is identical on both atoms. And because there is this known that dipole, it means it means that this molecule is non polar. B.ethylene has one triple bond.
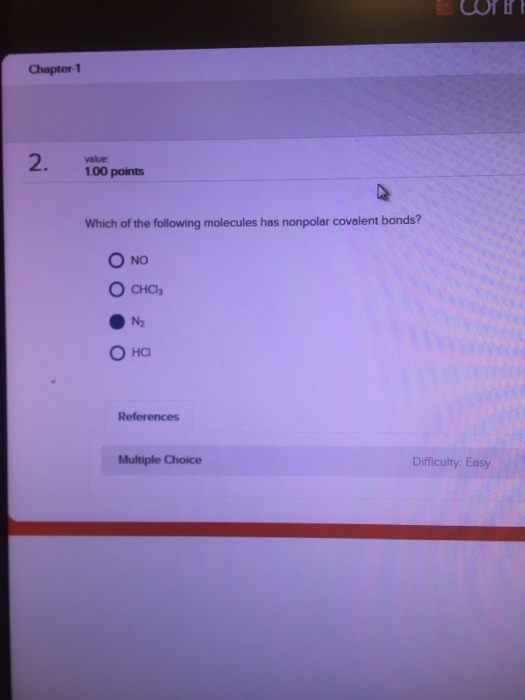
Be soluble in nonpolar solvents. A) becl 2 b) br 2 c) bf 3 d) ibr e) co 2 ans: The next molecule that we have is our dime on a fluoride.
 Source: chegg.com
Source: chegg.com
An example is the bf3. Which one of the following molecules is nonpolar? 1)a large nonpolar molecule is likely to be.
Also Read :