Has a strong conjugate base d. C 2 h 2 o 4 ) hydrofluoric acid (chemical formula:
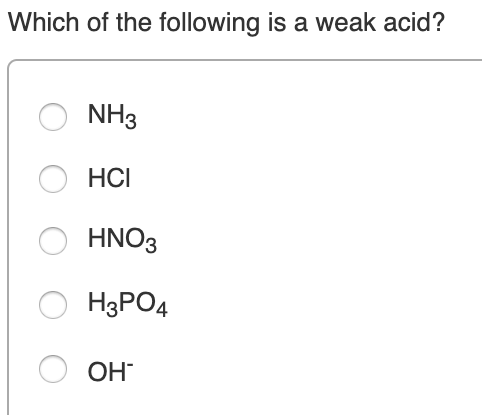
Which One Of The Following Is A Weak Acid. Is slightly dissociated in aqueous solution 27. Hcooh) acetic acid (chemical formula: Weak acids are mych more numerous than strong acids d. Ii) what is a weak acid?
 Which One Of The Following Is A Weak Acid?… | Clutch Prep From clutchprep.com
Which One Of The Following Is A Weak Acid?… | Clutch Prep From clutchprep.com
Related Post Which One Of The Following Is A Weak Acid?… | Clutch Prep :
Two acids �a� and �b� were kept in beakers. Weak acids are mych more numerous than strong acids d. (a) hno 3 (b) hi (c) hbr (d) hf (e) hclo 3 2. Acids capable of yielding mare than one hydronium ion per molecule are called polybasic acids, the dibasic, tribasic etc indicating the number of replaceable hydrogen.
I) of the two acids �a� and �b�, which is weak acid and which is strong acid?
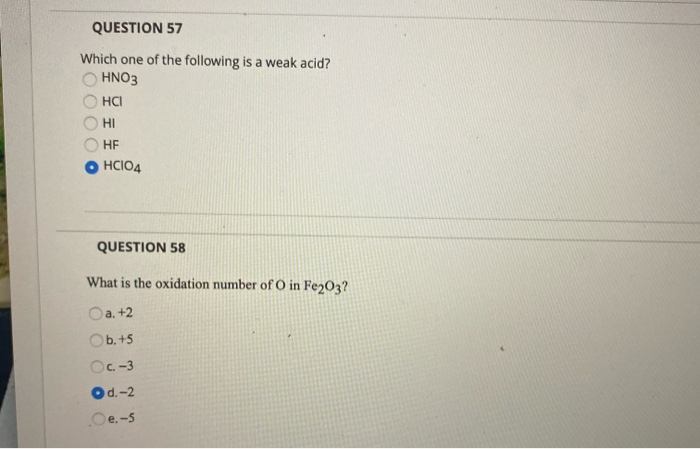
C 2 h 2 o 4 ) hydrofluoric acid (chemical formula: I) of the two acids �a� and �b�, which is weak acid and which is strong acid? Which of the following acids will have the strongest conjugate base? Which one of the following is an example of a weak acid? Which one of the following is a weak acid? Hclo4 free expert solution we’re being asked to identify which among the given acids is a weak acid.
 Source: numerade.com
Source: numerade.com
Which of the following are weak acids a) hf, hbr b) hi, hno3, hbr c) hi, hf d) hf e) none of the above Has a strong conjugate base d. Weak acids ionize only slightly in diute aqueous solution b.
 Source: pt.slideshare.net
Source: pt.slideshare.net
Acids capable of yielding mare than one hydronium ion per molecule are called polybasic acids, the dibasic, tribasic etc indicating the number of replaceable hydrogen. Which salt is not derived from a strong acid and a strong soluble base? Which of the following statements about weak acids is false?
 Source: bartleby.com
Source: bartleby.com
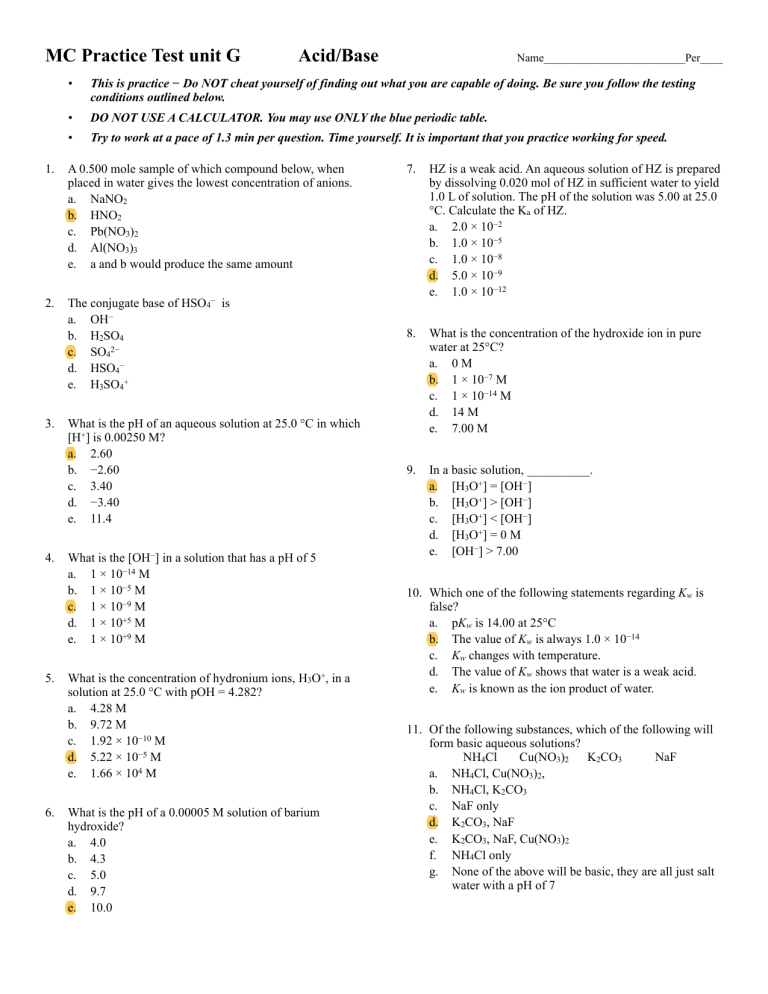
Ch 3 cooh) benzoic acid (chemical formula: Answer true or false for each of the following: Which of the following are weak acids a) hf, hbr b) hi, hno3, hbr c) hi, hf d) hf e) none of the above

Weak acids are mych more numerous than strong acids d. Which one of the following is a weak acid? (a) hno 3 (b) hi (c) hbr (d) hf (e) hclo 3 2.
 Source: thoughtco.com
Source: thoughtco.com
H c l , h 2 s o 4 and h n o 3 are strong acids. A weak acid is an acid which on dissociation in an aqueous solution produces low concentration of h+ ions. Iv) give one example of each.

Which one of the following is a weak acid? I) of the two acids �a� and �b�, which is weak acid and which is strong acid? (a) one mole of any acid will ionize completely in aqueous solution to produce one mole of h + ions.

Some common examples of weak acids are listed below. Which compound is a weak acid? Hcooh) acetic acid (chemical formula:
 Source: studylib.net
Source: studylib.net
Many weak acids are familiar to us in everyday use e. Which of the following acids will have the strongest conjugate base? Weak acids ionize only slightly in diute aqueous solution b.

Which of the following are weak acids a) hf, hbr b) hi, hno3, hbr c) hi, hf d) hf e) none of the above But carbonic acid h2co3, dissociate only partially. D) the conjugate base of a very weak acid is stronger than the conjugate base of a strong acid.

(a) one mole of any acid will ionize completely in aqueous solution to produce one mole of h + ions. Acids capable of yielding mare than one hydronium ion per molecule are called polybasic acids, the dibasic, tribasic etc indicating the number of replaceable hydrogen. I) of the two acids �a� and �b�, which is weak acid and which is strong acid?
 Source: clutchprep.com
Source: clutchprep.com
C 2 h 2 o 4 ) hydrofluoric acid (chemical formula: Hf) nitrous acid (chemical formula: Has a strong conjugate base d.
 Source: numerade.com
Source: numerade.com
D) the conjugate base of a very weak acid is stronger than the conjugate base of a strong acid. A strong acid is one that completely ionises in water to form a large amount of hydrogen ions whereas a weak acid only partially ionises in water and thus produces a small amount of hydrogen ions. So carbonic acid is the weakest among the above acids.

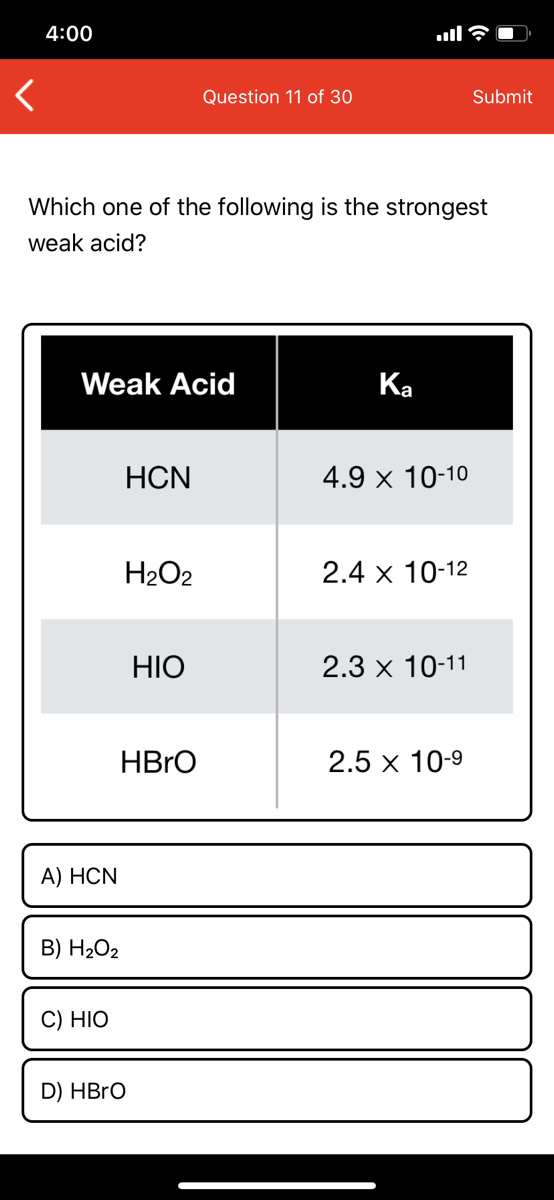
Strong mineral acids like h2so4, hcl and hno3 completely dissociate to h+ ions and the respective anions. Many weak acids are familiar to us in everyday use e. Consider the following acids and their dissociation constants:

(a) one mole of any acid will ionize completely in aqueous solution to produce one mole of h + ions. Strong mineral acids like h2so4, hcl and hno3 completely dissociate to h+ ions and the respective anions. 4 weeks, 1 day ago.

Two acids �a� and �b� were kept in beakers. A strong acid is one that completely ionises in water to form a large amount of hydrogen ions whereas a weak acid only partially ionises in water and thus produces a small amount of hydrogen ions. A weak acid is an acid which on dissociation in an aqueous solution produces low concentration of h+ ions.
 Source: chegg.com
Source: chegg.com
Which salt is not derived from a strong acid and a strong soluble base? Weak acids are weak electrolytes that don’t completely ionize but instead reach a state of equilibrium. In aqueous solution, lactic acid partially dissociates according to the following reaction:
 Source: clutchprep.com
Source: clutchprep.com
Click here👆to get an answer to your question ️ which compound is a weak acid? The ka values for weak acids are numbers that are greater than 1 c. Two acids �a� and �b� were kept in beakers.
 Source: chegg.com
Source: chegg.com
(a) hno 3 (b) hi (c) hbr (d) hf (e) hclo 3 2. Weak acids ionize only slightly in diute aqueous solution b. A strong acid is one that completely ionises in water to form a large amount of hydrogen ions whereas a weak acid only partially ionises in water and thus produces a small amount of hydrogen ions.
 Source: chegg.com
Source: chegg.com
C 6 h 5 cooh) oxalic acid (chemical formula: Which of the following statements about weak acids is false? Which of the following are weak acids a) hf, hbr b) hi, hno3, hbr c) hi, hf d) hf e) none of the above
 Source: chegg.com
Source: chegg.com
So carbonic acid is the weakest among the above acids. Which of the following statements about weak acids is false? (a) hno 3 (b) hi (c) hbr (d) hf (e) hclo 3 2.
Also Read :





