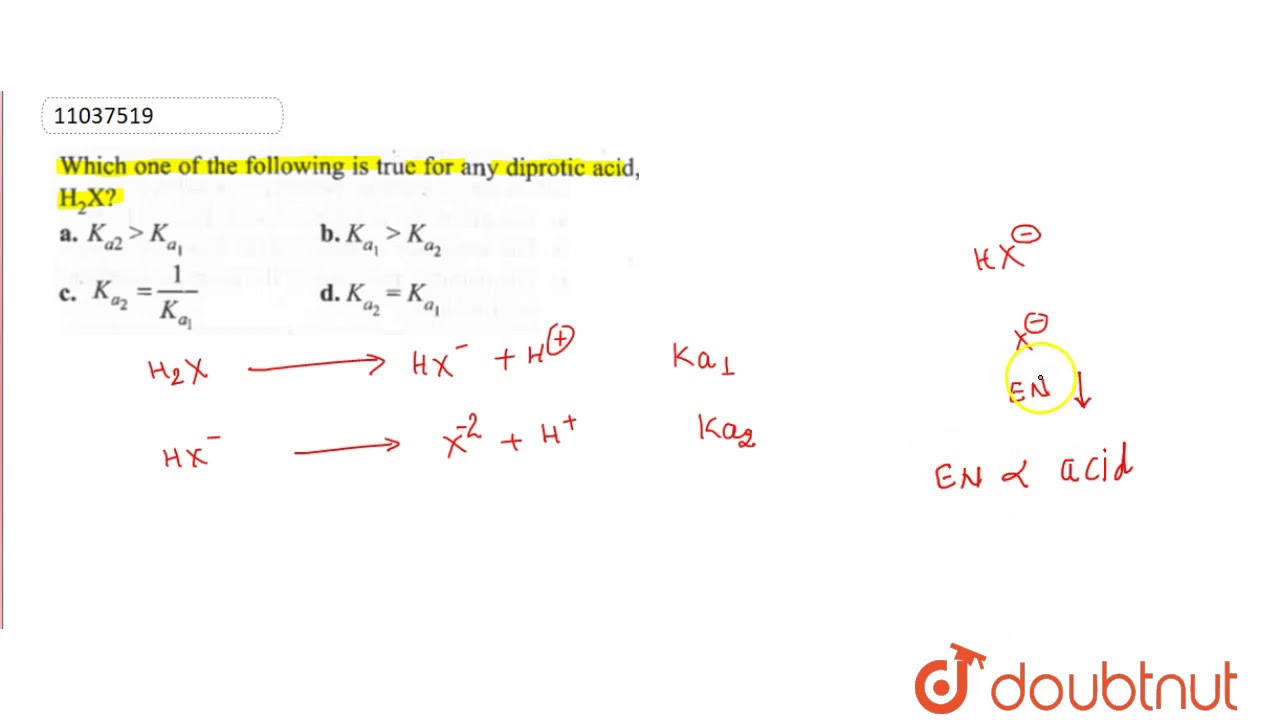
Shown below is a sample titration curve for a diprotic acid.note the two equivalence points. Which one of the following is true for any diprotic acid h2x ?
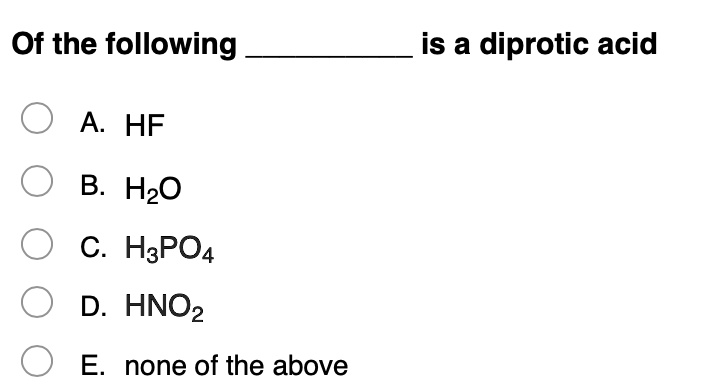
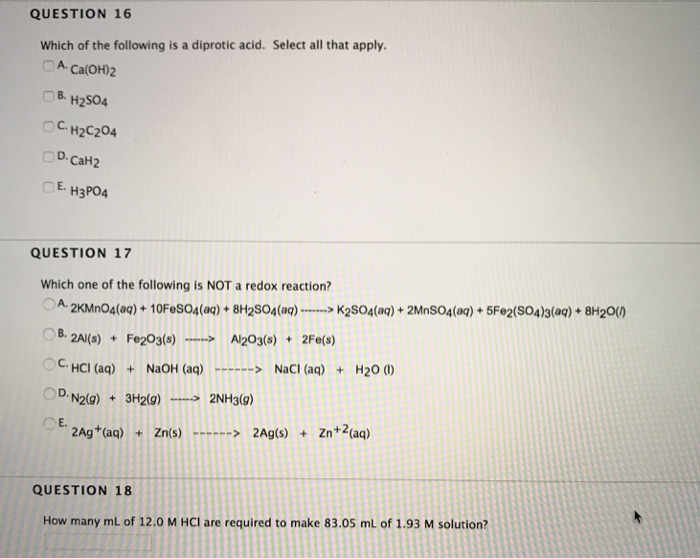
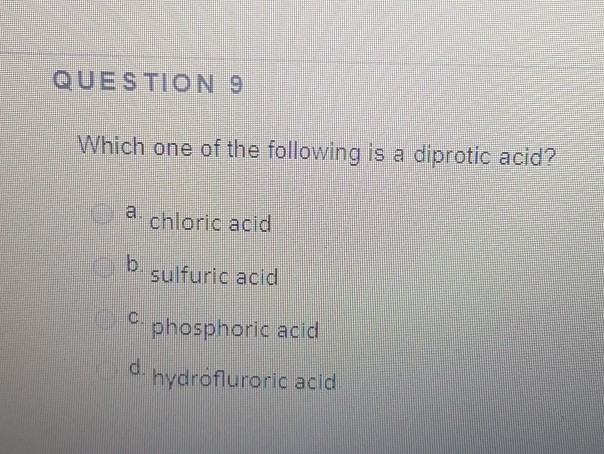
Which One Of The Following Is A Diprotic Acid. Phosphoric acid is tricrotic acid, it contains three hydrogen ions. Nitric acid is monoprotic acid,it contains one acidic hydrogen atom. A) nitric acid b) chloric acid c) phosphoric acid d) hydrofluoric acid e) sulfuric acid question 2 what is the oxidation state of xenon in xeo4? Hence, among the given oxoacids, six compounds are diprotic acids which are h 2 s o 4, h 3 p o 3, h 2 c o 3, h 2 s 2 o 7, h 2 c r o 4, h 2 s o 3.
 16.7 Polyprotic Acid Titrations - Youtube From youtube.com
16.7 Polyprotic Acid Titrations - Youtube From youtube.com
Related Post 16.7 Polyprotic Acid Titrations - Youtube :
(a) hypophosphorous acid is a diprotic acid (b) all oxoacids contain tetra Two hydrogen atoms are directly attached to the p. Among the given acids sulfuric acid is a diprotic acid. (a) ka1 = ka2 (b) ka1
(ii) h_(3)po_(3) is a diprotic acid.
(ii) h_(3)po_(3) is a diprotic acid. H2so4), a triprotic acid three moles of h+ (e.g. Which of the following is true for a solution of any diprotic acid 1 ph log2 h 3 from ch 302 at university of texas When a diprotic acid, is titrated with , the protons on the diprotic acid are generally removed one at a time, resulting in a ph curve that has the following generic shape a. Which is the correct statement for the given acids? Which one of the following is a diprotic acid?a.
 Source: chegg.com
Source: chegg.com
A diprotic acid is an acid that can donate two hydronium ions (h +). Which is the correct statement for the given acids? Phosphinic acid is a monoprotic acid as it contains one p − o h bond while phosphonic acid is a diprotic acid as it contains two p − o h bonds.
 Source: clutchprep.com
Source: clutchprep.com
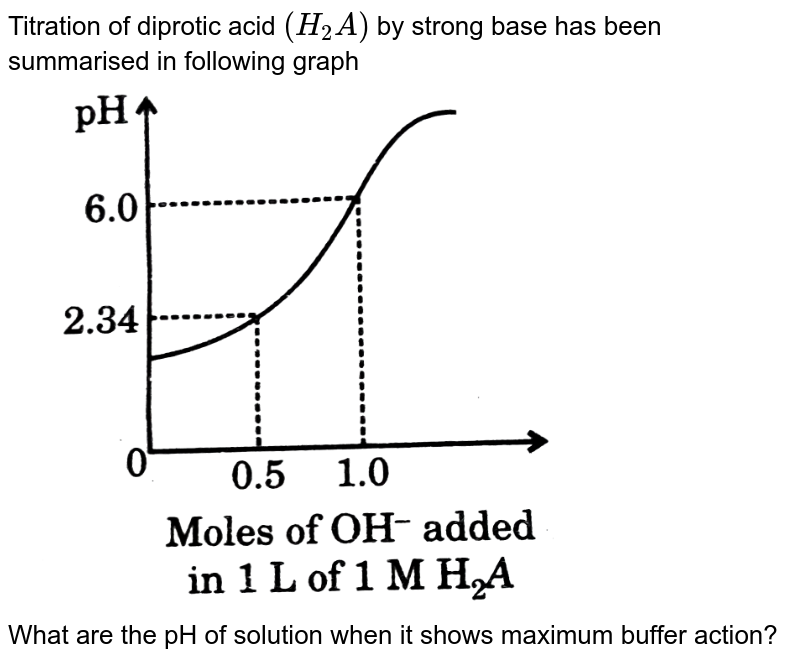
Which one of the following is a diprotic acid? Two hydrogen atoms are directly attached to the p. If the first equivalence point occurs at 100.0ml added, what volume of added corresponds to the second equivalence point
 Source: clutchprep.com
Source: clutchprep.com
Two hydrogen atoms are directly attached to the p. A diprotic acid is a type of polyprotic acid, which is an acid able to donate more than one proton per molecule. H2co3 and h2so3 are called diprotic acids, and h3po3 and h3po4 are called triprotic acids.
 Source: doubtnut.com
Source: doubtnut.com
Account for the h3po3 is a diprotic acid. Diprotic acids, such as sulfuric acid (h2so4), carbonic acid (h2co3), hydrogen sulfide (h2s), chromic acid (h2cro4), and oxalic acid (h2c2o4) have two acidic hydrogen atoms. Which one of the following is a diprotic acid?a.
 Source: toppr.com
Source: toppr.com
(a) phosphinic acid is a monoprotic acid while phosphonic acid is a diprotic acid. Which of the following statements is not valid for oxoacids of phosphorus ? A diprotic acid is an acid that can donate two hydronium ions (h +).
 Source: youtube.com
Source: youtube.com
When a diprotic acid is titrated with a strong base, and the ka1 and ka2 are significantly different, then the. A) nitric acid b) chloric acid c) phosphoric acid d) hydrofluoric acid e) sulfuric acid question 2 what is the oxidation state of xenon in xeo4? O hc question 4 which one of the following is a diprotic acid o phosphoric acid o chloric acid sulfuric acid o hydrofluroric acid o nitric acid.
 Source: numerade.com
Source: numerade.com
Phosphinic acid is a monoprotic acid as it contains one p − o h bond while phosphonic acid is a diprotic acid as it contains two p − o h bonds. Account for the h3po3 is a diprotic acid. When a diprotic acid, is titrated with , the protons on the diprotic acid are generally removed one at a time, resulting in a ph curve that has the following generic shape a.
 Source: studylib.net
Source: studylib.net
A) nitric acid b) chloric acid c) phosphoric acid d) hydrofluoric acid e) sulfuric acid question 2 what is the oxidation state of xenon in xeo4? A) b b) po c) si d) ge e) as (ii) h_(3)po_(3) is a diprotic acid.
 Source: chegg.com
Source: chegg.com
A diprotic acid will produce two moles of h+ (e.g. জলীয় দ্রবণে নিচের ডাইপ্রোটিক অ্যাসিডগুলির. Diprotic acids, such as sulfuric acid (h 2 so 4), carbonic acid (h 2 co 3), hydrogen sulfide (h 2 s), chromic acid (h 2 cro 4), and oxalic acid (h 2 c 2 o 4) have two acidic hydrogen atoms.

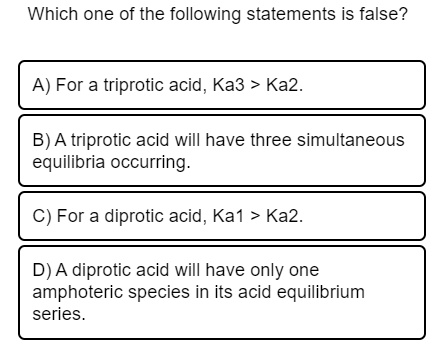
(a) ka1 = ka2 (b) ka1 Account for the following : Which of the following acids is a diprotic, weak acid?
 Source: numerade.com
Source: numerade.com
Two hydrogen atoms are directly attached to the p. Asked aug 9, 2019 in chemistry by studystudystudy. Which of the following acids is a diprotic, weak acid?
 Source: youtube.com
Source: youtube.com
H2so4), a triprotic acid three moles of h+ (e.g. A) nitric acid b) chloric acid c) phosphoric acid d) hydrofluoric acid e) sulfuric acid question 2 what is the oxidation state of xenon in xeo4? Which is the correct statement for the given acids?
(i) ammonia is a stronger base than phosphine. Which one of the following is a diprotic acid?a. A) b b) po c) si d) ge e) as
 Source: toppr.com
Source: toppr.com
When a diprotic acid is titrated with a strong base, and the ka1 and ka2 are significantly different, then the. A) phosphoric acid b) hydrochloric acid c) nitric acid d) sulfuric acid e) acetic acid A diprotic acid will produce two moles of h+ (e.g.

Notice that the plot has essentially two titration curves. Hence, among the given oxoacids, six compounds are diprotic acids which are h 2 s o 4, h 3 p o 3, h 2 c o 3, h 2 s 2 o 7, h 2 c r o 4, h 2 s o 3. Nitric acid is monoprotic acid,it contains one acidic hydrogen atom.
![Solved] Which One Of The Following Is The Best Representation Of The Titration | Quiz+](https://d2lvgg3v3hfg70.cloudfront.net/TB7800/11eac68a_1c4a_773e_a448_771f25945ba3_TB7800_00.jpg “Solved] Which One Of The Following Is The Best Representation Of The Titration | Quiz+") Source: quizplus.com
Which is the correct statement for the given acids? When a diprotic acid, is titrated with , the protons on the diprotic acid are generally removed one at a time, resulting in a ph curve that has the following generic shape a. A diprotic acid is an acid that can donate two hydronium ions (h +).
 Source: clutchprep.com
Source: clutchprep.com
Which of the following acids is a diprotic, weak acid? Hence, among the given oxoacids, six compounds are diprotic acids which are h 2 s o 4, h 3 p o 3, h 2 c o 3, h 2 s 2 o 7, h 2 c r o 4, h 2 s o 3. Account for the h3po3 is a diprotic acid.
 Source: researchgate.net
Source: researchgate.net
Account for the h3po3 is a diprotic acid. A) phosphoric acid b) hydrochloric acid c) nitric acid d) sulfuric acid e) acetic acid Which one of the following is a diprotic acid?a.
 Source: chegg.com
Source: chegg.com
A diprotic acid will produce two moles of h+ (e.g. Two hydrogen atoms are directly attached to the p. Which one of the following is a diprotic acid?
 Source: numerade.com
Source: numerade.com
A) +8 b) +6 c) +4 d) +2 e) 0 question 3 of the following, only _____ is not a metalloid. A) b b) po c) si d) ge e) as A) +8 b) +6 c) +4 d) +2 e) 0 question 3 of the following, only _____ is not a metalloid.
Also Read :