The most acidic compound is 1, while the least acidic compound is 5. A a ) ethyl acetoacetate
Which One Of The Following Compounds Is Most Acidic. Which one of the following compounds is most acidic? A a ) ethyl acetoacetate Oh ci oh 500 oh oh solution : That�s why is a strong acid among the given.
Related Post Solved Which One Of The Following Compounds Is Most Acidic? | Chegg.com :
And we know more stable the corresponding conjugate base of the acid,more acidic the acid is. Which of the following resonance structures contributes the most to the resonance hybrid: Option 1) option 2) option 3) option 4) The negative charge on oatom is destabilised as electron releasing aminogroup is present in para position.
A a ) ethyl acetoacetate
The negative charge on oatom is destabilised as electron releasing aminogroup is present in para position. To answer this question first we should know that how to check the acidic strength of any compound. The most stable conformation is 1, while the least stable conformation is 5. Which of the following is the most basic? And in (d) methyl group shows +i effect and. Phenols are much more acidic than alcohols due to the stabilisation of phenoxide ion be resonance.
 Source: numerade.com
Source: numerade.com
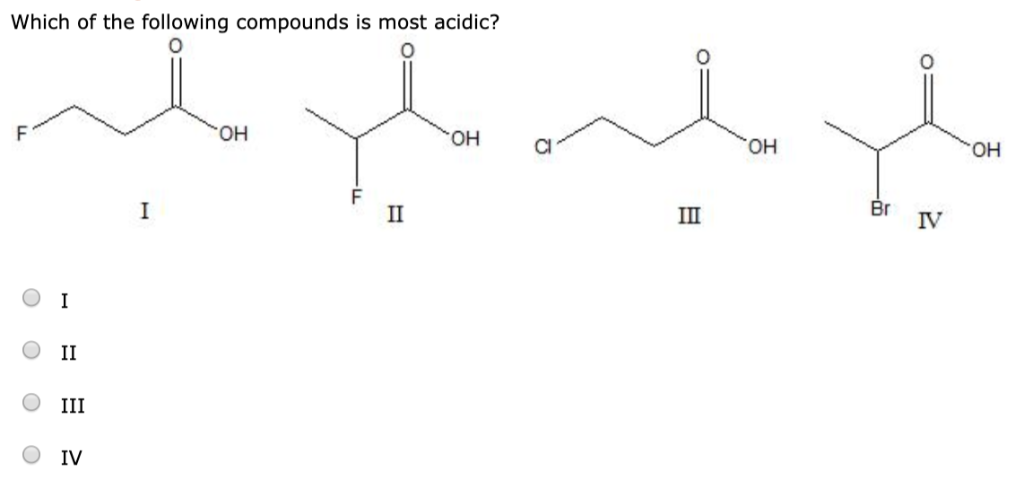
Which of the following compounds is most acidic? Which of the following compounds is most acidic? Which one of the following compounds is most acidic?
 Source: toppr.com
Source: toppr.com
Want to see this answer and more? Thus, compound (a), in acidic conditions, will most readily be dehydrated. The negative charge on oatom is destabilised as electron releasing aminogroup is present in para position.
 Source: questions-in.kunduz.com
Source: questions-in.kunduz.com
Which one of the following compounds has the most acidic nature? Acidic strength oh oh oh oh ci pk, = 8.48 9.02 9.38 9.95 answer Ii we can explain acidity of given compounds on the basis of stability of conjugate base, more stable the conjugate base, more acidic the acid and vice versa.
Hence, the compound which gives the most stable conjugate base will be the most acidic. Option 1) option 2) option 3) option 4) Acidic strength oh oh oh oh ci pk, = 8.48 9.02 9.38 9.95 answer
 Source: oneclass.com
Source: oneclass.com
Acidic strength oh oh oh oh ci pk, = 8.48 9.02 9.38 9.95 answer Option 1) option 2) option 3) option 4) Which one of the following compounds has the most acidic nature?
![Solved] Which Of The Following Compounds Is Most Acidic? | Course Hero](https://www.coursehero.com/qa/attachment/17318449/ “Solved] Which Of The Following Compounds Is Most Acidic? | Course Hero”) Source: coursehero.com
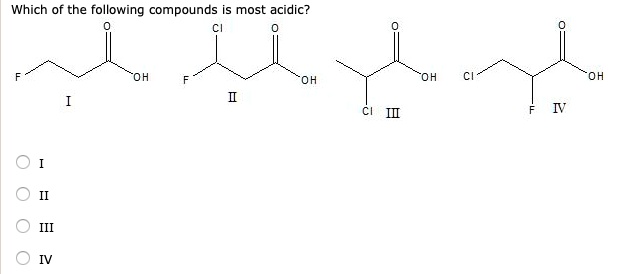
Which one of the following compounds possesses the most acidic hydrogen? Nitro group is highly electron withdrawing (by both conjugation as well as inductive effects). Which one of the following compounds possesses the most acidic hydrogen?
 Source: numerade.com
Source: numerade.com
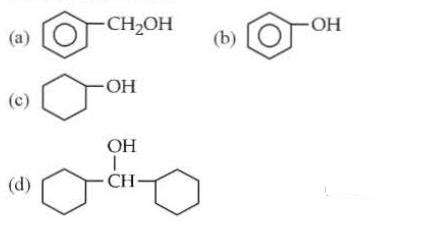
Which of the following compounds is most acidic? The most stable conformation is 1, while the least stable conformation is 5. Phenols are much more acidic than alcohols due to the stabilisation of phenoxide ion be resonance.
 Source: study.com
Source: study.com
Phenols are much more acidic than alcohols, due to the stabilisation of phenoxide ion by resonance questions from aipmt 2005 1. Check out a sample q&a here. Okay, so correct option will be c.

Which one of the following compounds is most acidic? Greater the stability and hence, easier the dehydration. Phenols are much more acidic than alcohols, due to the stabilisation of phenoxide ion by resonance questions from aipmt 2005 1.
Check out a sample q&a here. Option 1) option 2) option 3) option 4) Which of the following is the most basic?
 Source: chegg.com
Source: chegg.com
Option 1) option 2) option 3) option 4) Rank the following compounds in the trend requested. The most stable conformation is 1, while the least stable conformation is 5.
 Source: toppr.com
Source: toppr.com
The inhibition of resonance is most pronounced in cases of nitrobenzoic acids followed by fluorobenzoic acids. Which one of the following compounds possesses the most acidic hydrogen? Methyl groups tend to increase the electron density of the ring by hyperconjugation.
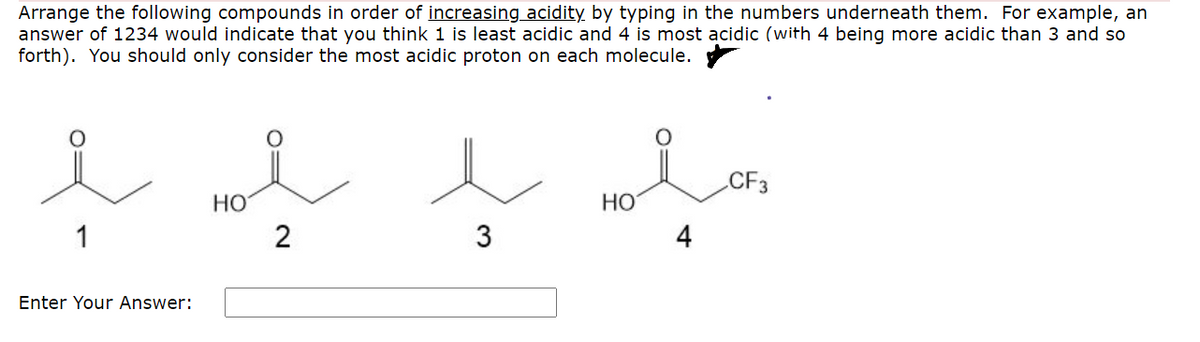 Source: bartleby.com
Source: bartleby.com
To explain stability of conjugate base , we can use ario rule. So firstly release 1 hydrogen from the compound. In case of p−aminophenol, deprotonation gives p−aminophenoxideion.
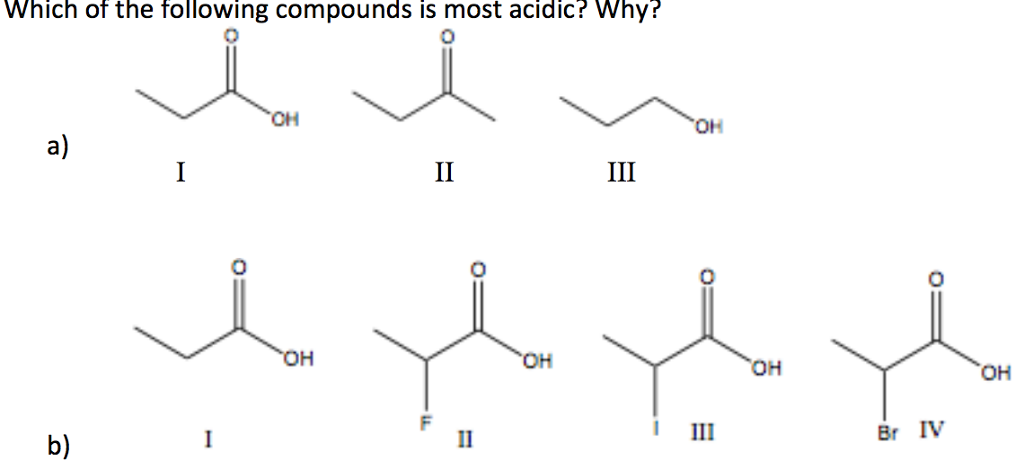 Source: chegg.com
Source: chegg.com
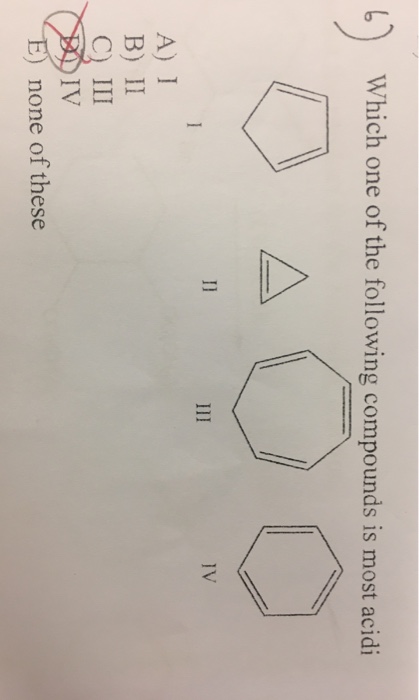
Rank the following compounds in the trend requested. Which one of the following compounds is most acidic? (a) (b) (c) (d) 57.

Which one of the following compounds has the most acidic nature? The cube shown in, 50.0 cm. To answer this question first we should know that how to check the acidic strength of any compound.
 Source: oneclass.com
Source: oneclass.com
Which one of the following compounds has the most acidic nature? Phenol is most acidic of all the given compounds. (a) (b) (c) (d) 57.

Nitro group is highly electron withdrawing (by both conjugation as well as inductive effects). Greater the stability and hence, easier the dehydration. Phenyl is an electron withdrawing substituent and hence increases acidity.

Acidic strength oh oh oh oh ci pk, = 8.48 9.02 9.38 9.95 answer Alkyne compounds which have acidic hydrogen atom are more acidic than alkynes which do not have acidic hydrogen atoms. Check out a sample q&a here.
![Solved] Which Of The Following Compounds Is Most Acidic? | Course Hero](https://www.coursehero.com/qa/attachment/12706312/ “Solved] Which Of The Following Compounds Is Most Acidic? | Course Hero”) Source: coursehero.com
The negative charge on oatom is destabilised as electron releasing aminogroup is present in para position. Okay, so correct option will be c. The most stable conformation is 1, while the least stable conformation is 5.
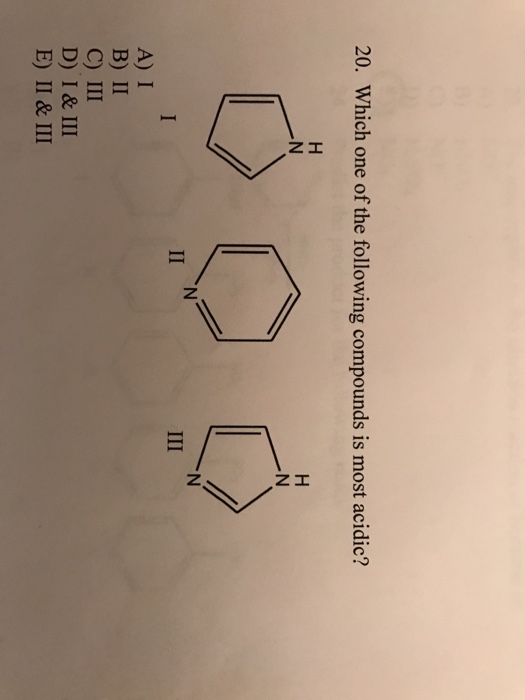
The most stable conformation is 1, while the least stable conformation is 5. Acidic strength oh oh oh oh ci pk, = 8.48 9.02 9.38 9.95 answer Phenol is most acidic of all the given compounds.
Also Read :