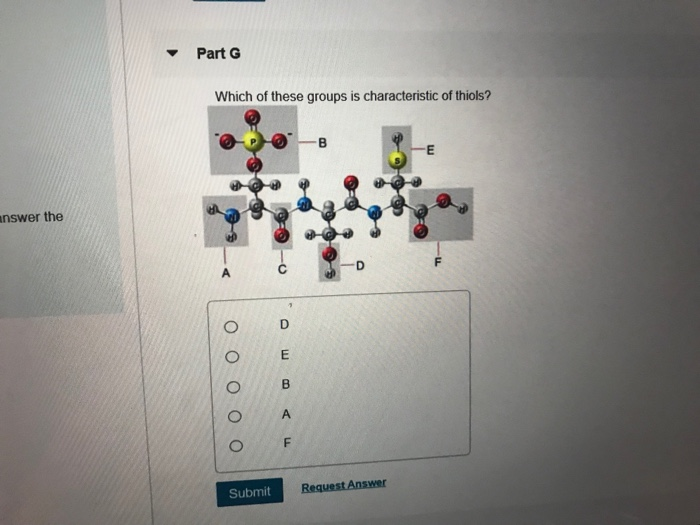
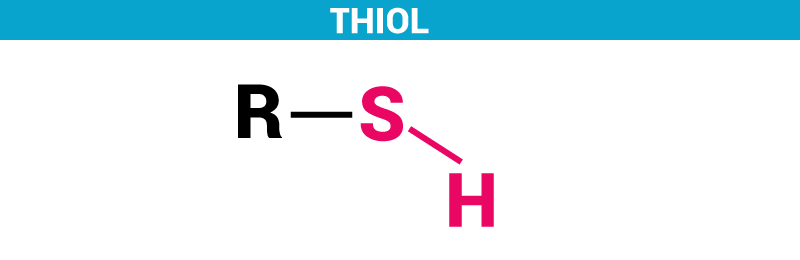
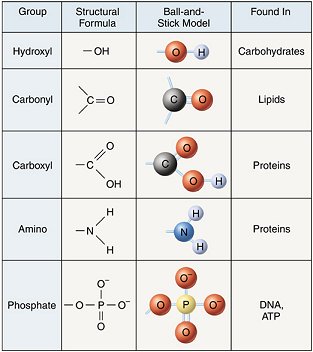
Due to the similar electronegativities of sulfur and hydrogen, thiols are less polar and have a lower dipole moment than the corresponding alcohols. The figure shows a big branched molecular model with six functional groups:
Which Of These Groups Is Characteristic Of Thiols. Which of these is a carbonyl group? Part h which of these groups plays a major role in energy transfer? What is another name for a condensation reaction? Which of these functional groups is characteristic of a ketone?
 Part A Which One Of These Is An Amino Group? | Course Hero From coursehero.com
Part A Which One Of These Is An Amino Group? | Course Hero From coursehero.com
Related Post Part A Which One Of These Is An Amino Group? | Course Hero :
Thiols are characterized by the presence of a sulfhydryl group. Which of these functional groups is characteristic of a ketone? Which of these groups plays a major role in energy transfer? Akin to the chemistry of alcohols, thiols form thioethers, thioacetals, and thioesters.
Part g which of these groups is characteristic of thiols?
Part h which of these groups plays a major role in energy transfer? Due to the similar electronegativities of sulfur and hydrogen, thiols are less polar and have a lower dipole moment than the corresponding alcohols. Part a which one of these is an amino group? A bond forms between the carboxyl functional group of one amino acid and the amino functional group of the other amino acid. Thiols are characterized by the presence of a sulfhydryl group. Unlock all answers please join to get access.
 Source: news-medical.net
Source: news-medical.net
Which of the following statements are true? Which of these is a carbonyl group? C, a carbonyl group in the middle of a carbon.
 Source: study.com
Source: study.com
Are isomers that differ in the covalent partnerships between their atoms. Are isomers in which one of the molecules contains an amino group and the other contains a phosphate group. Which of these is a carbonyl group.
 Source: thoughtco.com
Source: thoughtco.com
Molecules termed thiols are characterized by this group, which resembles a hydroxyl group. Which of these groups plays a major role in energy transfer? Which of these functional groups is characteristic of a ketone?
 Source: mdpi.com
Source: mdpi.com
Which of these functional groups is characteristic of a ketone? Which of the following statements are true? Which of these functional groups behaves as an acid?
 Source: chegg.com
Source: chegg.com
Sa ctivity and answer the o o o o o submit request answer part part 1. C, a carbonyl group in the middle of a carbon. Which of these functional groups behaves as an acid?
 Source: sciencedirect.com
Source: sciencedirect.com
Which of the functional groups behaves as an acid. Which of these is a carbonyl group? What functional group is characteristic of alcohol.
 Source: slideserve.com
Source: slideserve.com
Part h which of these groups plays a major role in energy transfer? C, a carbonyl group in the middle of a carbon. Correct thiols are characterized by the presence of a sulfhydryl group.
 Source: onlinelibrary.wiley.com
Source: onlinelibrary.wiley.com
Which of these groups is characteristic of thiols? A bond forms between the carboxyl functional group of one amino acid and the amino functional group of the other amino acid. C, a carbonyl group in the middle of a carbon.
 Source: youtube.com
Source: youtube.com
Which of these groups plays a major role in energy transfer? Unlock all answers please join to get access. Which of these functional groups is characteristic of a ketone?
 Source: sciencedirect.com
Source: sciencedirect.com
Which of these functional groups is characteristic of a ketone? E, thiols are characterized by the presence of a sulfhydryl group. Part g which of these groups is characteristic of thiols?
 Source: byjus.com
Source: byjus.com
Which of these is a carbonyl group. Which of these groups is characteristic of thiols? Are isomers in which one of the molecules contains an amino group and the other contains a phosphate group.
 Source: alyseq.blogspot.com
Source: alyseq.blogspot.com
Which of these is a carbonyl group? E, thiols are characterized by the presence of a sulfhydryl group. B d e f a d e c.
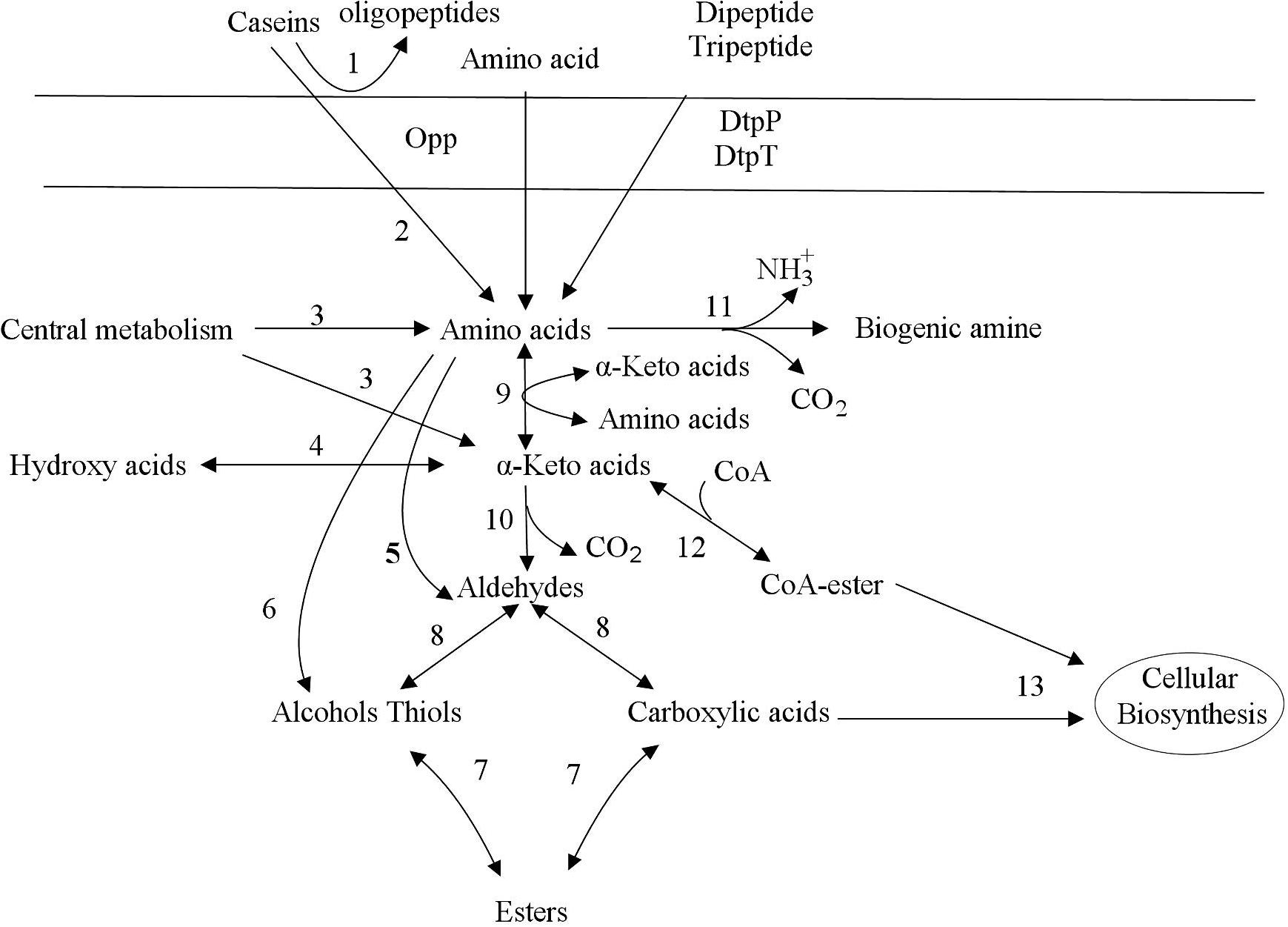 Source: frontiersin.org
Source: frontiersin.org
Learn this topic by watching functional groups concept videos. What is the characteristic feature of all alkynes? Differ in the arrangement of their molecules about a double bond.
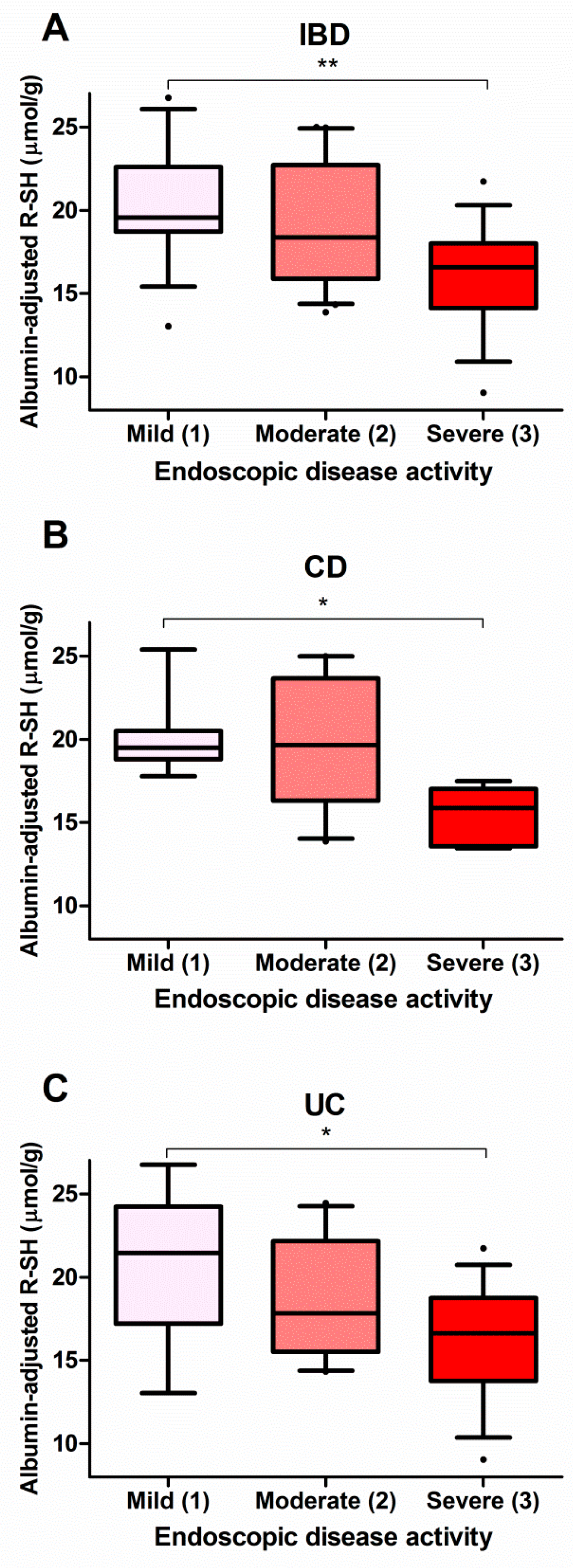 Source: mdpi.com
Source: mdpi.com
Molecules termed thiols are characterized by this group, which resembles a hydroxyl group. Which of these groups plays a major role in energy transfer? What is a characteristic of a ketone?
 Source: masteringbiologyquiz.com
Source: masteringbiologyquiz.com
Share this link with a friend: Which of these groups is characteristic of thiols? Group b represents an atom of phosphorus, which is bonded to four oxygen atoms.
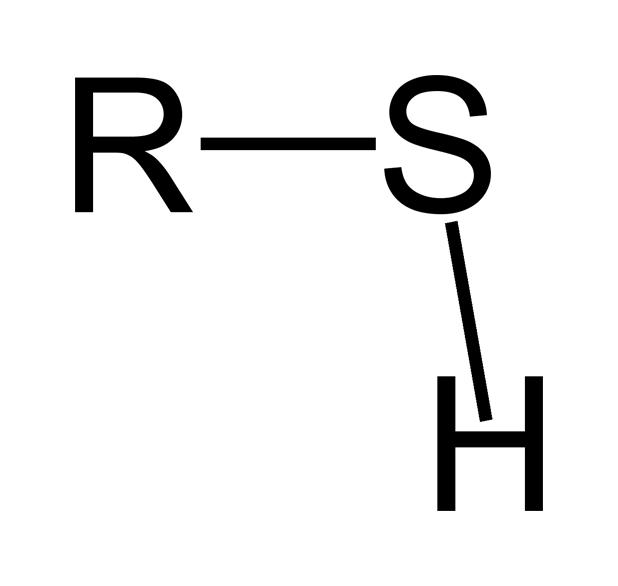 Source: wikidoc.org
Source: wikidoc.org
Differ in their molecular formulas. Sulfhydrl group thiols are characterized by the presence of a sulfhydryl group. Due to the similar electronegativities of sulfur and hydrogen, thiols are less polar and have a lower dipole moment than the corresponding alcohols.
 Source: quizlet.com
Source: quizlet.com
Which of these groups is characteristic of thiols. Which of these is a carbonyl group? The figure shows a big branched molecular model with six functional groups:
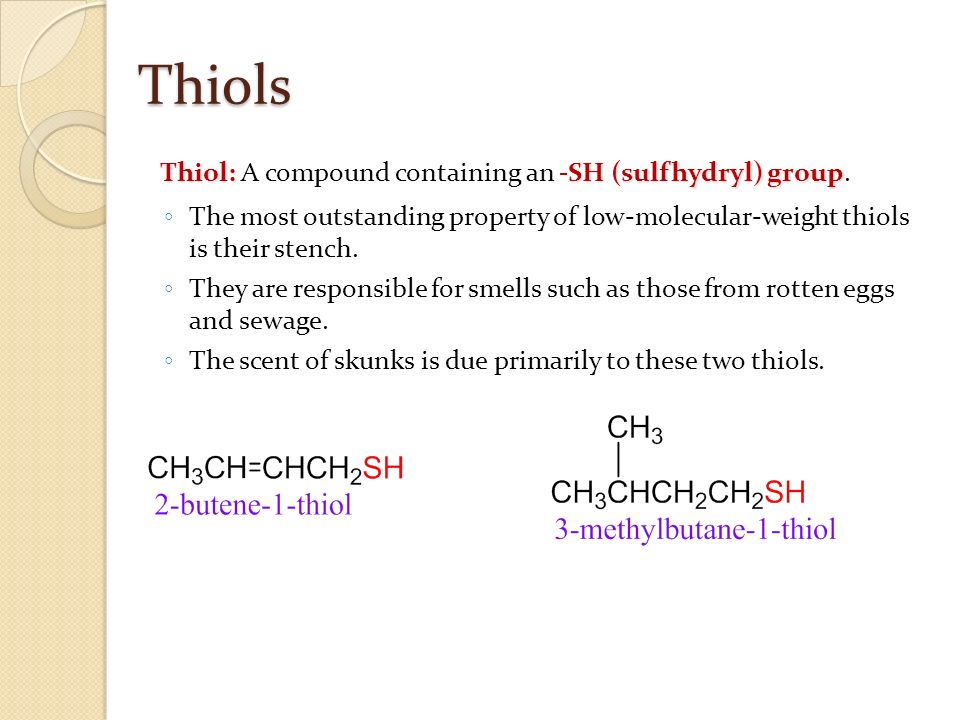 Source: slideplayer.com
Source: slideplayer.com
Which of these groups is characteristic of thiols? Which group plays a major role in energy? Which of the following statements are true?
 Source: nature.com
Source: nature.com
Another characteristic feature of most thiols is that they can act as reducing agents. Akin to the chemistry of alcohols, thiols form thioethers, thioacetals, and thioesters. Part a which one of these is an amino group?
 Source: coursehero.com
Source: coursehero.com
Which of these is a carbonyl group? Learn this topic by watching functional groups concept videos. What is the characteristic feature of all alkynes?
Also Read :