Which of the following statements about nad+ is true? Grading policy chapter 3 key concept quiz question 1 part a which of the following statements about carbon skeletons is true?
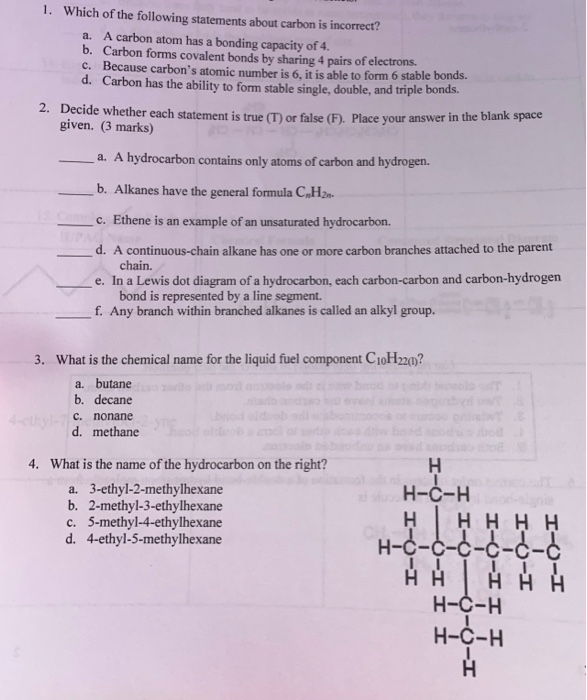
Which Of The Following Statements About Carbon Skeletons Is True. They contain more hydrogen than unsaturated fats having the same number of carbon atoms. Knowledge/comprehension refer to figure 9.1 to answer the following questions. A snake eats a mouse. Which of the following statements about carbon skeletons is true?
 Which Of The Following Is True Of Carbon? … | Clutch Prep From clutchprep.com
Which Of The Following Is True Of Carbon? … | Clutch Prep From clutchprep.com
Related Post Which Of The Following Is True Of Carbon? … | Clutch Prep :
How many electron pairs does carbon share to complete its valence shell? Two of these statements are true. Multiple answers are accepted for this question select. Which of the following statements about nad+ is true?
Mass increases as each animal adds mass.
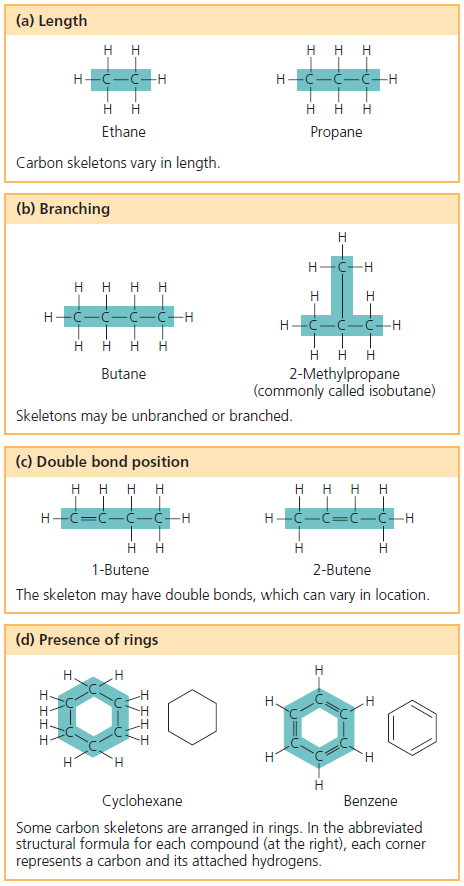
Which of the following is true of carbon? (2) the net effect of the urea cycle is the production of urea. Which of the following statements is true of the amino acids in the amino acid pool? C) it is highly electronegative. Why is carbon so important in biology? See figure 3.1b in your textbook, which displays the varied characteristics of carbon skeletons.

B) it can form a maximum of three covalent bonds with other elements. Mass is lost as it moves from animal to animal. Antibonding mos have electron density mainly outside the space between the two nuclei.
 Source: chegg.com
Source: chegg.com
D) none of the statements is true. Mass decreases because the animals do not consume all parts. When placed in water it a) would function only as an acid because of the carboxyl group.
 Source: coursehero.com
Source: coursehero.com
Which of the following statements about nad+ is true? They generally solidify at room temperature. All antibonding mos are higher in energy than the atomic orbitals of which they are composed.
 Source: chegg.com
Source: chegg.com
Carbon has six electrons, four of which are valence electrons. A carbon atom forms 4 covalent bonds. How many electron pairs does carbon share to complete its valence shell?
 Source: chegg.com
Source: chegg.com
Then, an owl eats the snake. It is the attachments that differentiate molecules. See figure 3.1b in your textbook, which displays the varied characteristics of carbon skeletons.

Which of the following statements is/are true as to the location in pyruvate of labeled carbons if glucose molecules labeled (in separate experiments) with 14c (radioactive carbon) at each position of the carbon skeleton proceed through the glycolytic pathway. Carbon skeletons may take the shape of a ring which of the following is incorrect pairing of a kingdom of an organism and the nutrient source of that organism D) would function as both an acid and a base.

All of the following statements about nah+ are true except: B) it can form a maximum of three covalent bonds with other elements. Which of the following statements about nad+ is true?

Carbon skeletons may be arranged in rings. Which of the following statements is/are true as to the location in pyruvate of labeled carbons if glucose molecules labeled (in separate experiments) with 14c (radioactive carbon) at each position of the carbon skeleton proceed through the glycolytic pathway. How many electron pairs does carbon share to complete its valence shell?
 Source: clutchprep.com
Source: clutchprep.com
All of the following statements concerning saturated fats are true. C) they can have very long carbon skeletons. And the breakdown of glucose.
 Source: chegg.com
Source: chegg.com
Which of the following statements about nad+ is true? Why is carbon so important in biology? It can form both polar and non polar bonds.
 Source: numerade.com
Source: numerade.com
A snake eats a mouse. A) in the absence of nad+, glycolysis can still function b) nad+ has more chemical energy than nadh c) nad+ can donate electrons for use in oxidative phosphorylation d) nad+ is reduced to nadh during glycolysis, pyruvate oxidation, and the citric acid cycle Which of the following statements about this system is true?
 Source: coursehero.com
Source: coursehero.com
A carbon atom forms 4 covalent bonds. Carbon has six electrons, four of which are valence electrons. All antibonding mos are higher in energy than the atomic orbitals of which they are composed.
 Source: chegg.com
Source: chegg.com
Multiple answers are accepted for this question 0 multiple. They generally solidify at room temperature. How many oxygen molecules (o2) are required each time a molecule of glucose (c6h12o6) is completely oxidized to carbon dioxide and water via aerobic respiration?
 Source: bartleby.com
Source: bartleby.com
It can form a variety of carbon skeletons and host functional groups. Carbon has six electrons, four of which are valence electrons. Antibonding mos have electron density mainly outside the space between the two nuclei.
 Source: numerade.com
Source: numerade.com
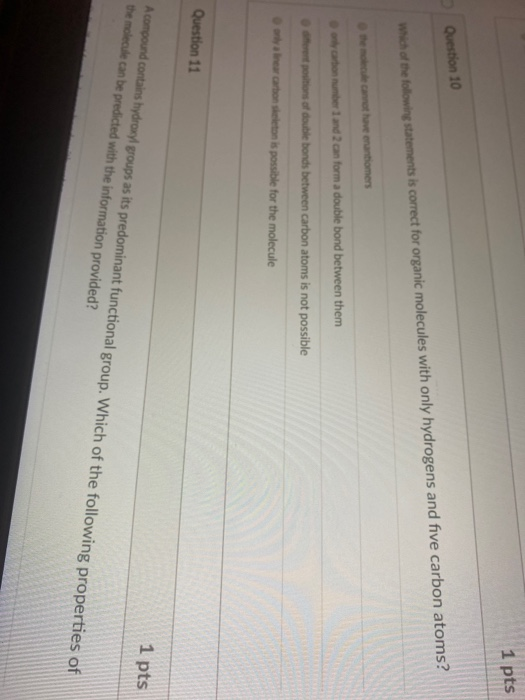
Multiple answers are accepted for this question select. A carbon skeleton is covalently bonded to both an amino group and a carboxyl group. Which of the following statements about carbon skeletons is true?
 Source: chegg.com
Source: chegg.com
It can form both polar and nonpolar bonds. Which of the following statements about multiple bonds is true? Why is carbon so important in biology?
 Source: numerade.com
Source: numerade.com
D) they have a lot of electrons associated with hydrogen. Carbon (atomic number 6) has electronic configuration 1s22s22p4 it has 4 valence electrons which are shared with other atoms to form 4 covalent bonds. D) would function as both an acid and a base.

C) only one of the statements is true. Which of the following is true of carbon? How many electron pairs does carbon share to complete its valence shell?
 Source: chegg.com
Source: chegg.com
In which form do the carbon skeletons of amino acids enter the common catabolic pathway? B) by varying the number of double bonds between carbon atoms. Carbon skeletons may take the shape of a ring which of the following is incorrect pairing of a kingdom of an organism and the nutrient source of that organism
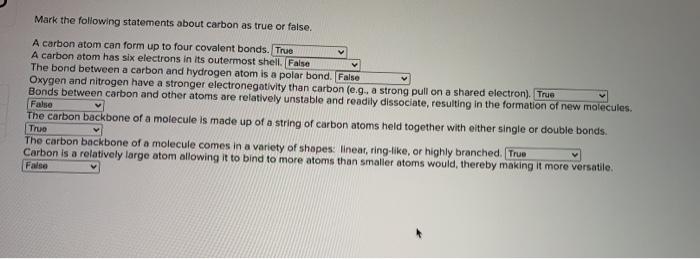
Which of the following is true of carbon? All of the following statements about nah+ are true except: How many oxygen molecules (o2) are required each time a molecule of glucose (c6h12o6) is completely oxidized to carbon dioxide and water via aerobic respiration?
Also Read :