The reactions in which succinate is converted to oxaloacetate are, in order a. Which of the following chemical equations describes a dehydration reaction?
Which Of The Following Reactions Is A Dehydration Reaction. When you see the word dehydration, the first thing that may come to mind is �losing water� or �lacking water.dehydration synthesis is classified as a type of chemical reaction. Updated on june 25, 2019. Which of the following chemical equations describes a dehydration reaction? D) dehydration reactions assemble polymers, and hydrolysis reactions break down polymers.
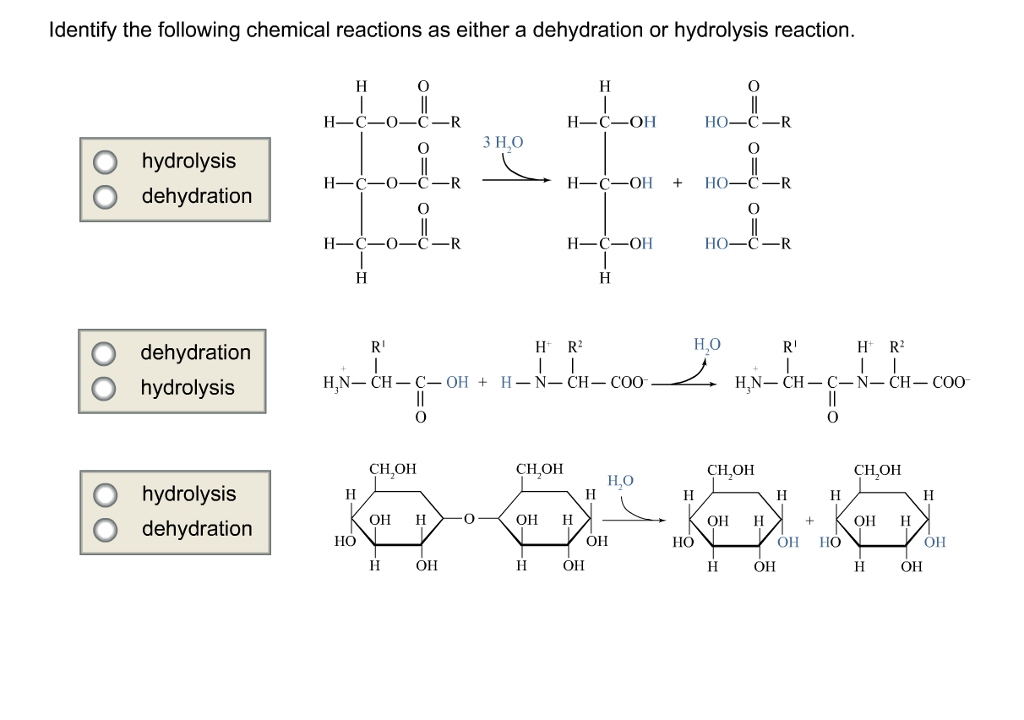 Solved Identify The Following Chemical Reactions As Either A | Chegg.com From chegg.com
Solved Identify The Following Chemical Reactions As Either A | Chegg.com From chegg.com
Related Post Solved Identify The Following Chemical Reactions As Either A | Chegg.com :
According to zaitsev�s rule, dehydration is regioselective and the more highly substituted alkene is formed (when a mixture of constitutional isomers is possible). A dehydration reaction is a chemical reaction in which a reactant loses a water molecule. A dehydration reaction is a chemical reaction between two compounds where one of the products is water. The general equation for an addition reaction:
Dehydration synthesis is a type of chemical reaction that involves the combining of reacting molecules to make a large molecule, following the loss of.
Which one is a hydrolysis reaction? A condensation, a dehydration, and an oxidative decarboxylation An oxidation, a dehydration, and an oxidation b. A + b → c. For example, two monomers may react where a hydrogen (h) from one monomer binds to a hydroxyl group (oh) from the other monomer to form a dimer and a water molecule (h2o). Updated on june 25, 2019.
 Source: sahay.guru
Source: sahay.guru
For example formation of the peptide from amino acids is a dehydration synthesis. Hcl + nahco3 → nacl + h2co3 a) dehydration synthesis. Which one is a hydrolysis reaction?
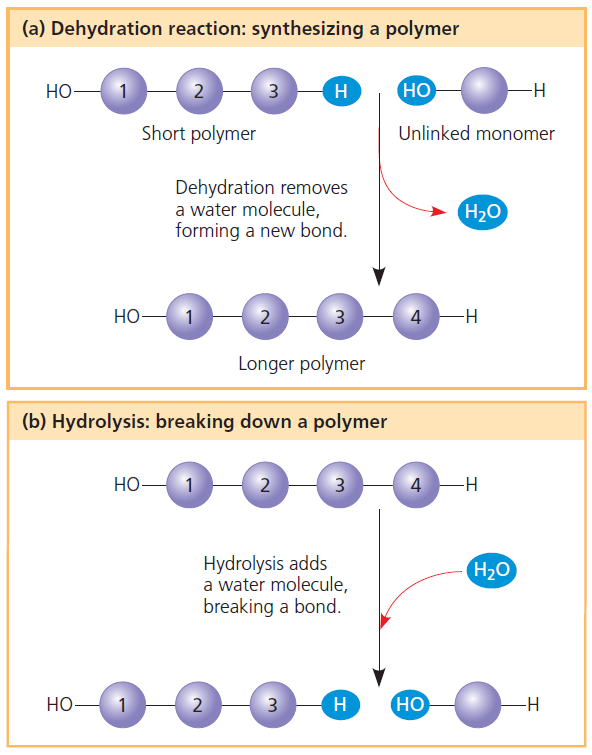 Source: clutchprep.com
Source: clutchprep.com
A chemical reaction between two compounds that produces water is a dehydration reaction. Since each step occurs with the elimination of water molecules, therefore the reaction is called a dehydration reaction and it results in the formation of new substance so it is named as a dehydration synthesis reaction. Multiple choice o a+b+h2o → c a b+c+ h20 a+h2o →.
 Source: study.com
Source: study.com
Which of the following reactions is a dehydration reaction? Construct a reaction coordinate diagram for the reaction. An oxidation, a dehydration, and an oxidation b.
 Source: chegg.com
Source: chegg.com
An oxidation, a dehydration, and an oxidation b. The 1st is fast and endothermic. Explore the definition and examples of a dehydration reaction and discover the difference between.
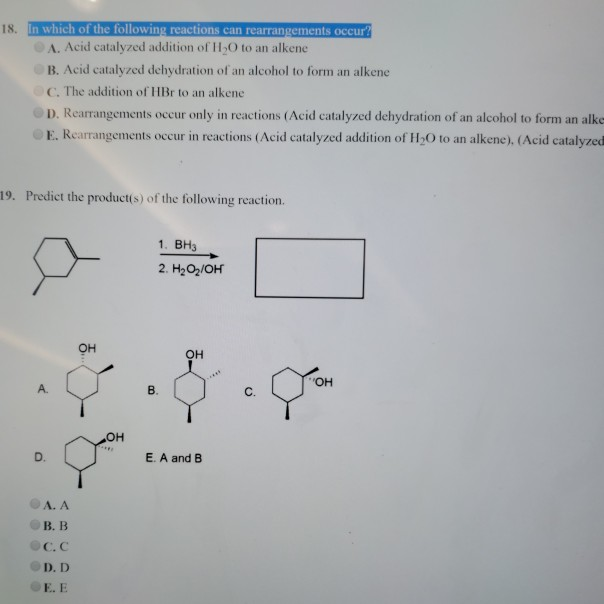 Source: chegg.com
Source: chegg.com
Updated on june 25, 2019. Updated on june 25, 2019. Construct a reaction mechanism that accounts for the product proposed.

Three successive oxidation reactions c. An oxidative decarboxylation, a dehydration, and a condensation d. If a, b, and c are molecules and c is a larger molecule than a and b, which of the two reactions is a dehydration reaction?
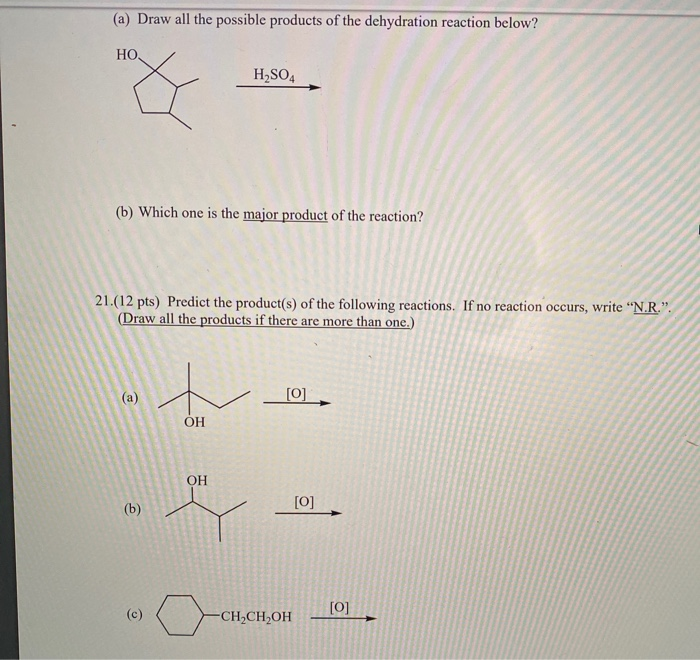 Source: chegg.com
Source: chegg.com
Which of the following is an exergonic reaction? Since each step occurs with the elimination of water molecules, therefore the reaction is called a dehydration reaction and it results in the formation of new substance so it is named as a dehydration synthesis reaction. Which of the following reactions is a dehydration reaction?
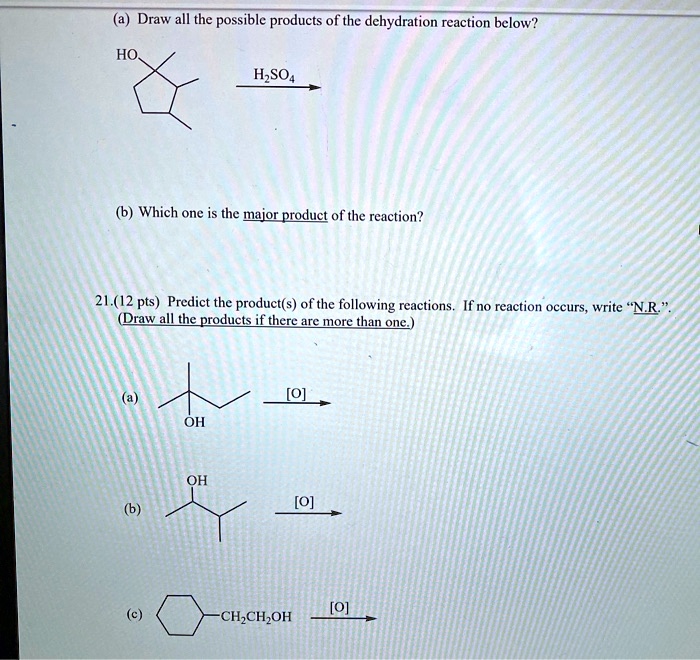 Source: numerade.com
Source: numerade.com
A dehydration reaction is a condensation reaction which involve condensation of 2 molecules an. Note that the reaction has three steps: 10) identify the following reaction:
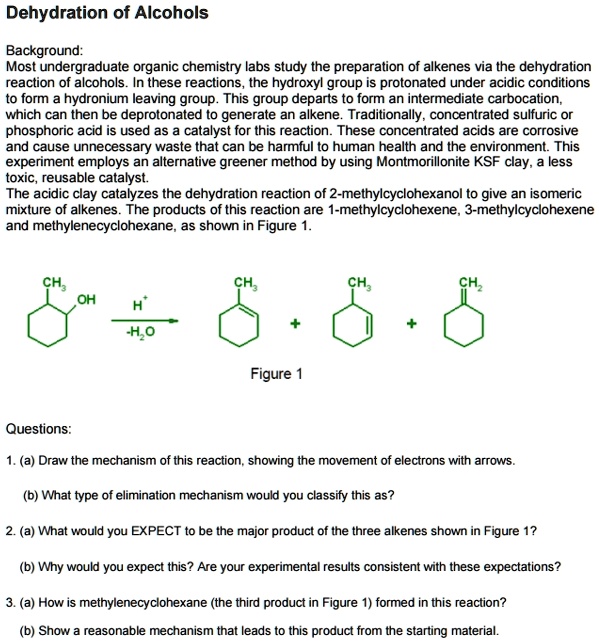 Source: numerade.com
Source: numerade.com
Carbohydrates, proteins, and nucleic acids are built up and broken down via these types of reactions, although the monomers involved are different in each case. A dehydration reaction is a condensation reaction which involve condensation of 2 molecules an. Which one is a hydrolysis reaction?
 Source: slideplayer.com
Source: slideplayer.com
For example formation of the peptide from amino acids is a dehydration synthesis. An elimination reaction occurs when a reactant is broken up into two products. A dehydration reaction is a chemical reaction between two compounds where one of the products is water.
 Source: chegg.com
Source: chegg.com
In chemistry, a dehydration reaction (a.k.a. For example, two monomers may react where a hydrogen (h) from one monomer binds to a hydroxyl group (oh) from the other monomer to form a dimer and a water molecule (h2o). What is a dehydration condensation reaction?
 Source: chegg.com
Source: chegg.com
Hcl + nahco3 → nacl + h2co3 a) dehydration synthesis. 9) identify the following reaction: Hcl + nahco3 → nacl + h2co3 a) dehydration synthesis.
 Source: youtube.com
Source: youtube.com
Construct a reaction mechanism that accounts for the product proposed. Three successive oxidation reactions c. In chemistry, a dehydration reaction (a.k.a.
 Source: chegg.com
Source: chegg.com
If a, b, and c are molecules and c is a larger molecule than a and b, which of the two reactions is a dehydration reaction? At room temperature, the reaction does not occur. Condensation reaction), also known as zimmer’s hydrogenesis, is a conversion that involves the loss of water from the reacting molecule or ion.
 Source: www2.chemistry.msu.edu
Source: www2.chemistry.msu.edu
For example, if two reactants are combined where a hydrogen from one reactant binds to a hydroxyl group from the other reactant, it can produce a dimer and a water molecule. Many biochemical reactions occur through dehydration synthesis mechanism. Hcl + nahco3 → nacl + h2co3 a) dehydration synthesis.

Which of the following reactions is a dehydration reaction? Construct a reaction coordinate diagram for the reaction. Since each step occurs with the elimination of water molecules, therefore the reaction is called a dehydration reaction and it results in the formation of new substance so it is named as a dehydration synthesis reaction.

A chemical reaction between two compounds that produces water is a dehydration reaction. Note that the reaction has three steps: An elimination reaction occurs when a reactant is broken up into two products.
 Source: chegg.com
Source: chegg.com
For example, if two reactants are combined where a hydrogen from one reactant binds to a hydroxyl group from the other reactant, it can produce a dimer and a water molecule. Lactose + h2o → glucose + galactose a) dehydration synthesis reaction b) hydrolysis reaction c) exchange reaction d) reversible reaction e) ionic reaction. D) dehydration reactions assemble polymers, and hydrolysis reactions break down polymers.
 Source: researchgate.net
Source: researchgate.net
For example, two monomers may react where a hydrogen (h) from one monomer binds to a hydroxyl group (oh) from the other monomer to form a dimer and a water molecule (h2o). Dehydration reactions split water molecules and add hydroxyl groups to polymers, and hydrolysis reactions remove hydroxyl groups from polymers. In chemistry, a dehydration reaction is a conversion that involves the loss of water from the reacting molecule or ion.dehydration reactions are common processes, the reverse of a hydration reaction.common dehydrating agents used in organic synthesis include sulfuric acid and alumina.
 Source: chegg.com
Source: chegg.com
Three successive oxidation reactions c. An oxidation, a dehydration, and an oxidation b. When you see the word dehydration, the first thing that may come to mind is �losing water� or �lacking water.dehydration synthesis is classified as a type of chemical reaction.
Also Read :