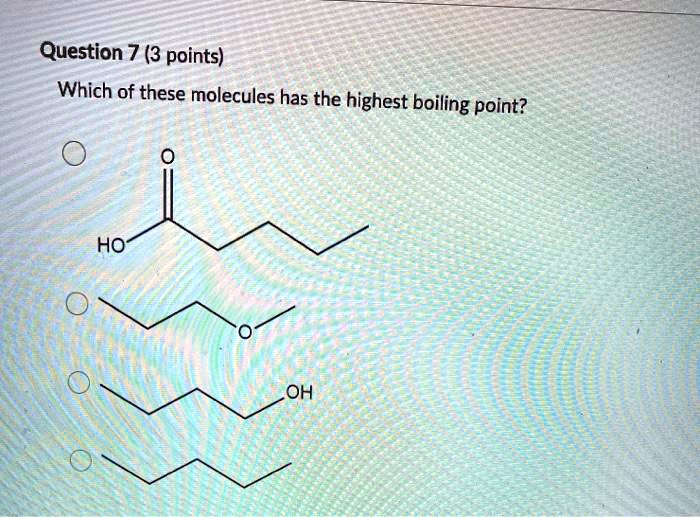
Hence, we can say acetic acid has the highest boiling point in given options. We can see that the largest carboxylic acid from our answer choices is pentanoic acid.
Which Of The Following Molecules Has The Highest Boiling Point. So the one with the highest molar mass will be the highest boiling point. This would result in a higher temperature at which boiling would occur. Ch4 = nonpolar molecule = london forces = lowest boiling point. The hydrogen bond has stronger intermolecular forces.
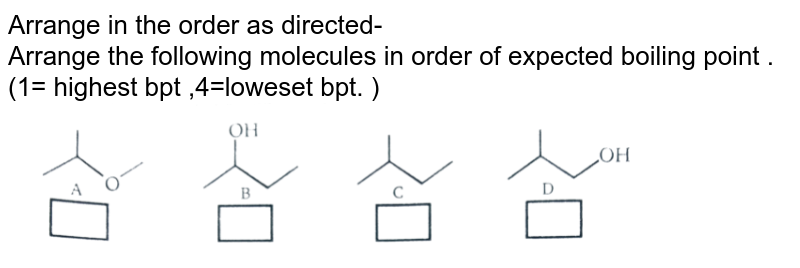 Arrange In The Order As Directed - Arrange The Following Molecu From amp.doubtnut.com
Arrange In The Order As Directed - Arrange The Following Molecu From amp.doubtnut.com
Related Post Arrange In The Order As Directed - Arrange The Following Molecu :
(d) the smaller the deviation from ideal gas behavior. The compound with the highest intermolecular forces will have the highest boiling point. Ionic compounds typically have high. As branching increases boiling point decreases.
Hcl will have a lower boiling point than hf since f is more electronegative than cl and possess a greater degree of hydrogen bonding.
Hydrogen bonding is the strongest intermolecular force. He, ne and xe are nobel gases. Notice c2 is the same for all answers with the other molecules being h, f, cl, br, i. Water has the highest boiling point because water is a strong dipole and the molecules are interconnected by hydrogen bonds. Which of the following has the highest boiling point? (b) the lower the boiling point.
 Source: bartleby.com
Source: bartleby.com
It all depends on the type and strength of the intermolecular forces between the molecules. What compound has the highest boiling point? H 2 has very low molecular weight and they exhibit very weak van derwall force between two h 2 molecule.
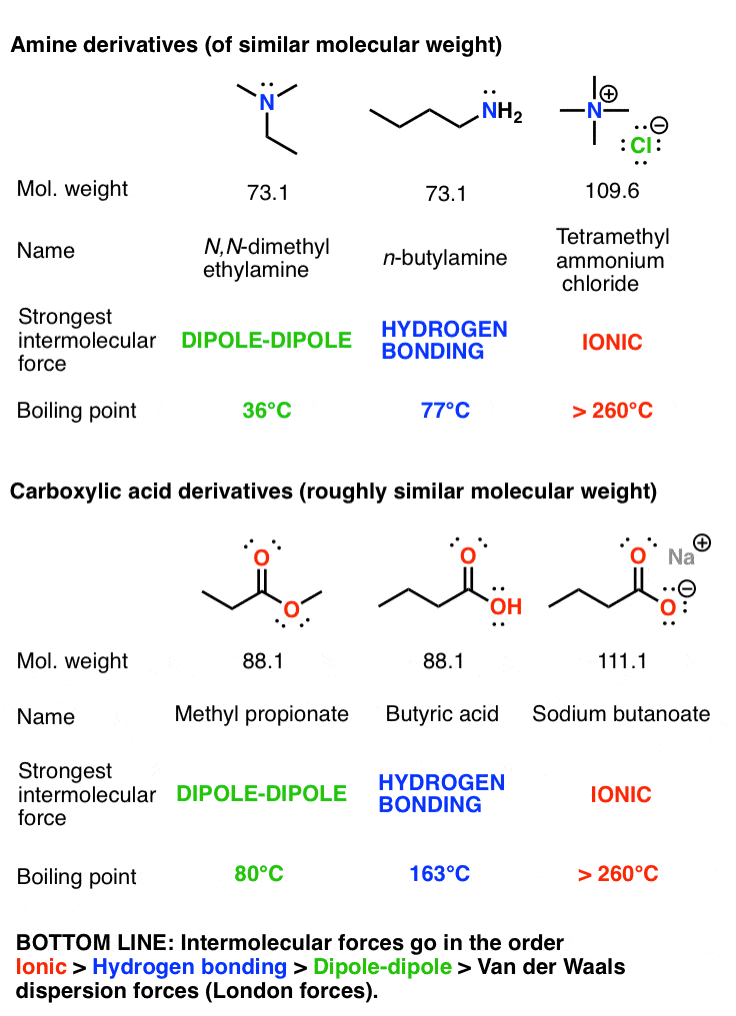 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
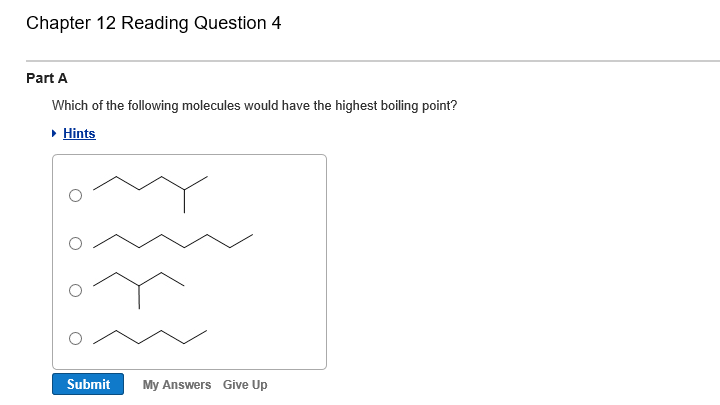
Bigger molecules will have stronger london dispersion forces. (a) ch4 (b) he (c) hf (d) cl2 3. Hence c4 h9 clstraight chain molecule with the highest number of carbons has the highest boiling point.
![Solved] Which Of The Following Molecules Has The Highest Boiling Point? Select One: Q A. Ch36H1Ch3 I? | Course Hero](https://www.coursehero.com/qa/attachment/2811568/ “Solved] Which Of The Following Molecules Has The Highest Boiling Point? Select One: Q A. Ch36H1Ch3 I? | Course Hero”) Source: coursehero.com
Of the following substances, _____ has the highest boiling point. F2, cl2, br2, i2 which substance has the highest/lowest melting/boiling point, etc. Hence c4 h9 clstraight chain molecule with the highest number of carbons has the highest boiling point.
 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
Straight chain alkyl halides have greater boiling point than their isomers. Q4 rank the following molecule according to their increasing stability. (c) the higher the vapor pressure.
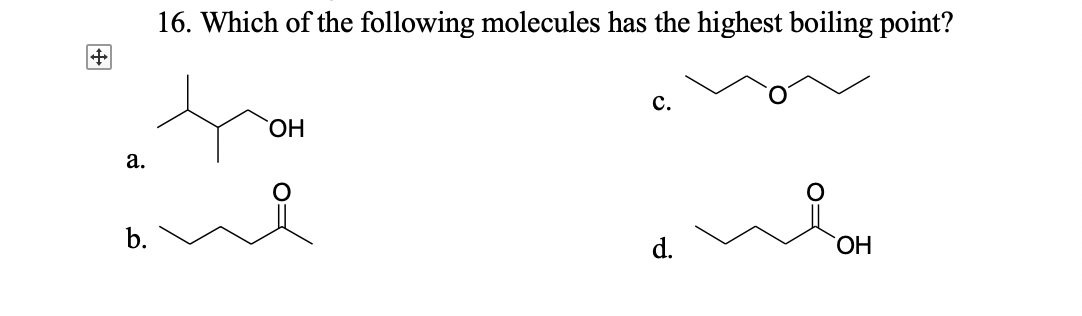 Source: bartleby.com
Source: bartleby.com
Straight chain alkyl halides have greater boiling point than their isomers. It all depends on the type and strength of the intermolecular forces between the molecules. Which substance has the highest boiling point?
 Source: studylib.net
Source: studylib.net
Which of the following has the highest boiling point? It shows that hi has the higher boiling point. This problem has been solved!
 Source: youtube.com
Source: youtube.com
Which of the following has the highest boiling point? (d) the smaller the deviation from ideal gas behavior. We can see that the largest carboxylic acid from our answer choices is pentanoic acid.
 Source: amp.doubtnut.com
Source: amp.doubtnut.com
The more sphere like the molecule, the lower its surface area will be and the fewer intermolecular van der waals interactions will operate. Hence break the bond is very difficult. This problem has been solved!
 Source: studylib.net
Source: studylib.net
In this case, hcl, hbr and hi all have dipoles, but ldf forces appear to be more important in determining the boiling point Boiling points increase as the number of carbons is increased. (a) the higher the boiling point.
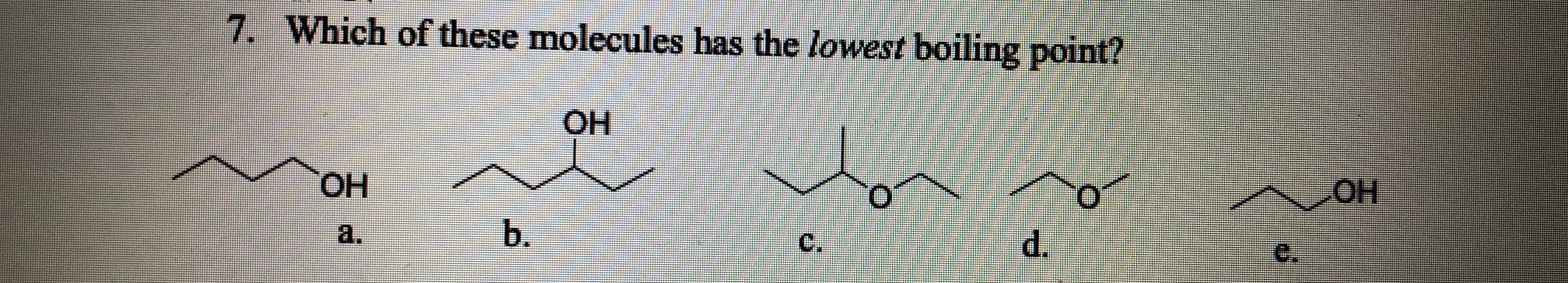 Source: bartleby.com
Source: bartleby.com
(b) the lower the boiling point. Which of the following has the highest boiling point? Where 1 is the lowest boiling point and 4 is the highest boiling point.
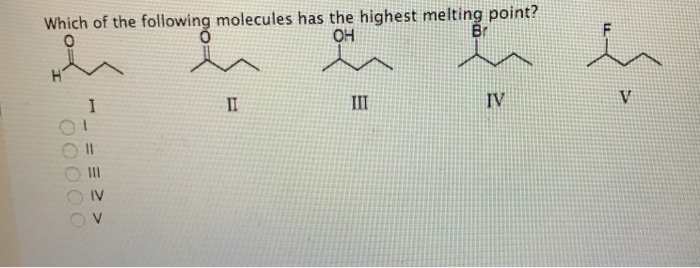
Arrange the compounds in order of increasing boiling point. So, they have lower boiling point than that of methane. A)c2cl6 b)c2br6 c)c2h6 d)c2f6 e)c2i6 i know its e, but.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
Boiling point is the temperature at which the vapor pressure of a liquid is equal to the external pressure surrounding the liquid. (a) the higher the boiling point. Which of the following has the highest boiling point?
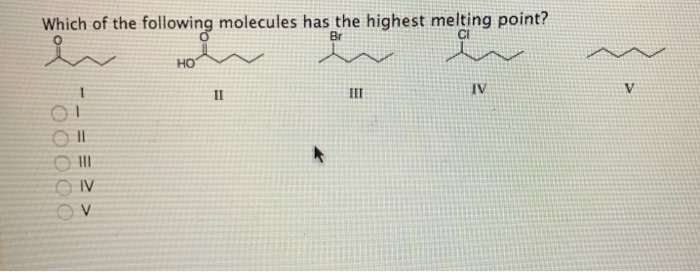
Of the following substances, _____ has the highest boiling point. Due to strong intermolecular forces, the molecules of hf will be tightly packed in a lattice. The more sphere like the molecule, the lower its surface area will be and the fewer intermolecular van der waals interactions will operate.
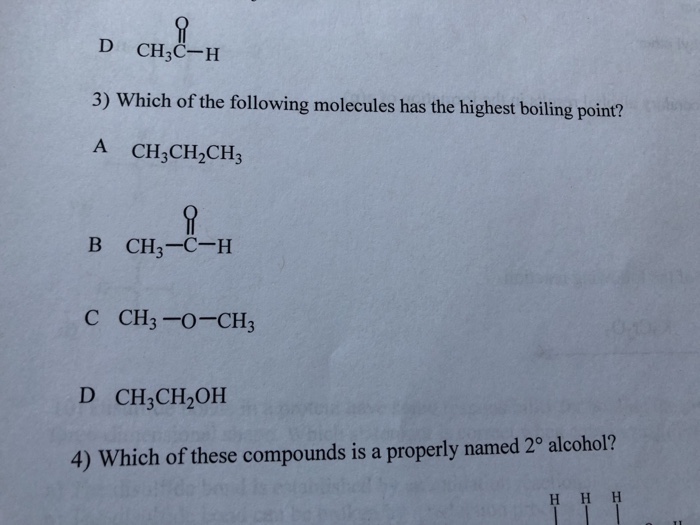
Hence, we can say acetic acid has the highest boiling point in given options. Where 1 is the lowest boiling point and 4 is the highest boiling point. Boiling points increase as the number of carbons is increased.
 Source: chegg.com
Source: chegg.com
It all depends on the type and strength of the intermolecular forces between the molecules. Correspondingly, i2 will have the highest boiling point and f2 will have the lowest boiling point. Where 1 is the lowest boiling point and 4 is the highest boiling point.
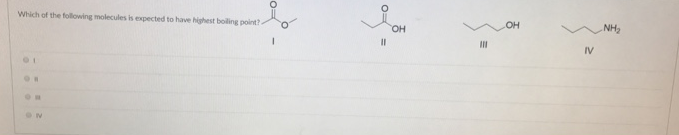 Source: clutchprep.com
Source: clutchprep.com
So, they have lower boiling point than that of methane. Ch4 = nonpolar molecule = london forces = lowest boiling point. It all depends on the type and strength of the intermolecular forces between the molecules.
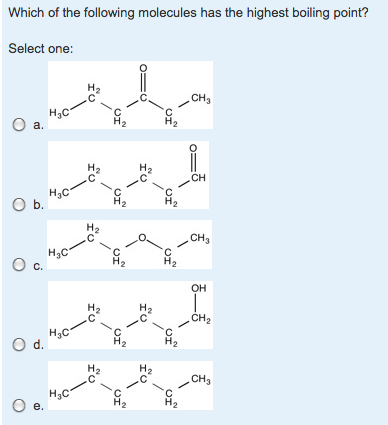 Source: chegg.com
Source: chegg.com
In this case, hcl, hbr and hi all have dipoles, but ldf forces appear to be more important in determining the boiling point Which of the following has the highest boiling point? We can see that the largest carboxylic acid from our answer choices is pentanoic acid.
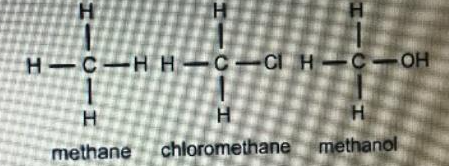 Source: study.com
Source: study.com
Hence break the bond is very difficult. As branching increases boiling point decreases. I know that the highest boiling point has to do with which has the strongest intermolecular force.
 Source: chegg.com
Source: chegg.com
The hydrogen bond has stronger intermolecular forces. What compound has the highest boiling point? Boiling point is the temperature at which the vapor pressure of a liquid is equal to the external pressure surrounding the liquid.
 Source: numerade.com
Source: numerade.com
(a) ch4 (b) he (c) hf (d) cl2 3. Hence c4 h9 clstraight chain molecule with the highest number of carbons has the highest boiling point. Water in fact has the highest boiling point because although its individual hydrogen bonds are not as strong as hydrogen fluoride�s, the fact there are twice as many (two h instead of one) means the total strength of intermolecular forces between water molecules is greater than that of hydrogen fluoride, and so has the highest boiling point.
Also Read :