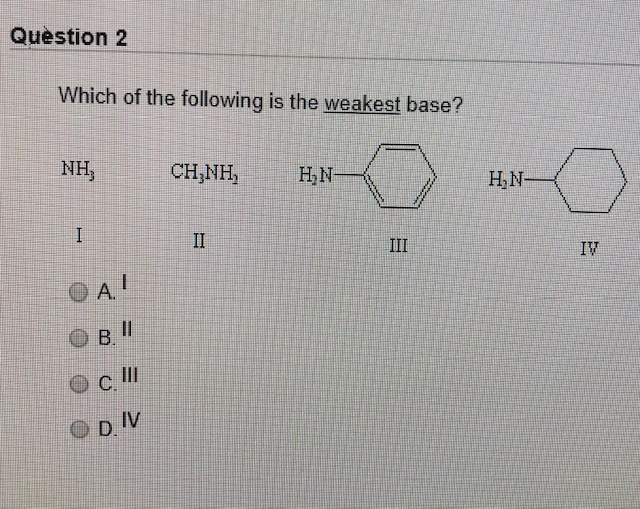
Let's list the anion or base, the conjugate acid and the pka. Here some of you may think that cyclohexylamine is showing strong basicity whether this was also attached with the ring.
Which Of The Following Is The Weakest Base. B) loss of a proton from an acid forms its conjugate base. C) gain of a proton by an acid forms its conjugate base. This means compound must have a strong lattice. Which of the following is the weakest base?
 Solved:question 42 Among The Following Anions; Which One Is The Weakest Base? Oh Sh From numerade.com
Solved:question 42 Among The Following Anions; Which One Is The Weakest Base? Oh Sh From numerade.com
Related Post Solved:question 42 Among The Following Anions; Which One Is The Weakest Base? Oh Sh :
Zn(oh) 2 is a weak base. (c) ammonia is the weakest reducing agent as well as the weakest base among group 15 hydrides. If you got any suggestions to improve this website, please feel free to send message on pkmcqs. If the conjugate acid is a weak acid (high pka), then the anion is a strong base.
Hence, zn(oh) 2 is the weakest base among the given options.
(d) ammonia is the strongest reducing agent and the. Which of the following is the weakest base? Therefore s the weakest base. The decreasing order of basicity of given hydroxides are as follows: The strongest acid among these will give weak base. The correct answer is (d) nh4oh.
 Source: oneclass.com
Source: oneclass.com
Taking it away from f is too hard. (a)ammonia is the weakest reducing agent and the strongest base among group 15 hydrides. Which of the following is the weakest base??
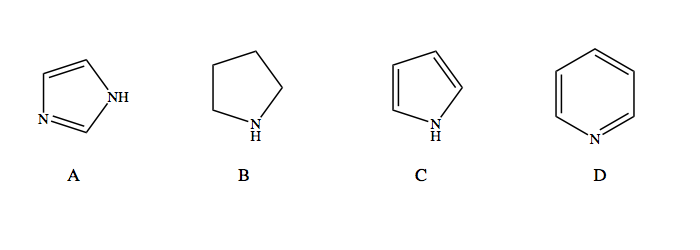 Source: clutchprep.com
Source: clutchprep.com
Chemistry mcqs, lioh , koh , naoh , rboh. Hf > h2o > nh3 > ch4. Which of the following statements is true.
 Source: youtube.com
Source: youtube.com
An aqueous sobution of hz is prepared by dissolving 0.020 mol of hz in sufficient water to h2 is. D) more than one choice is correct. So it does not readily donate an electron pair making it the weakest lewis base.
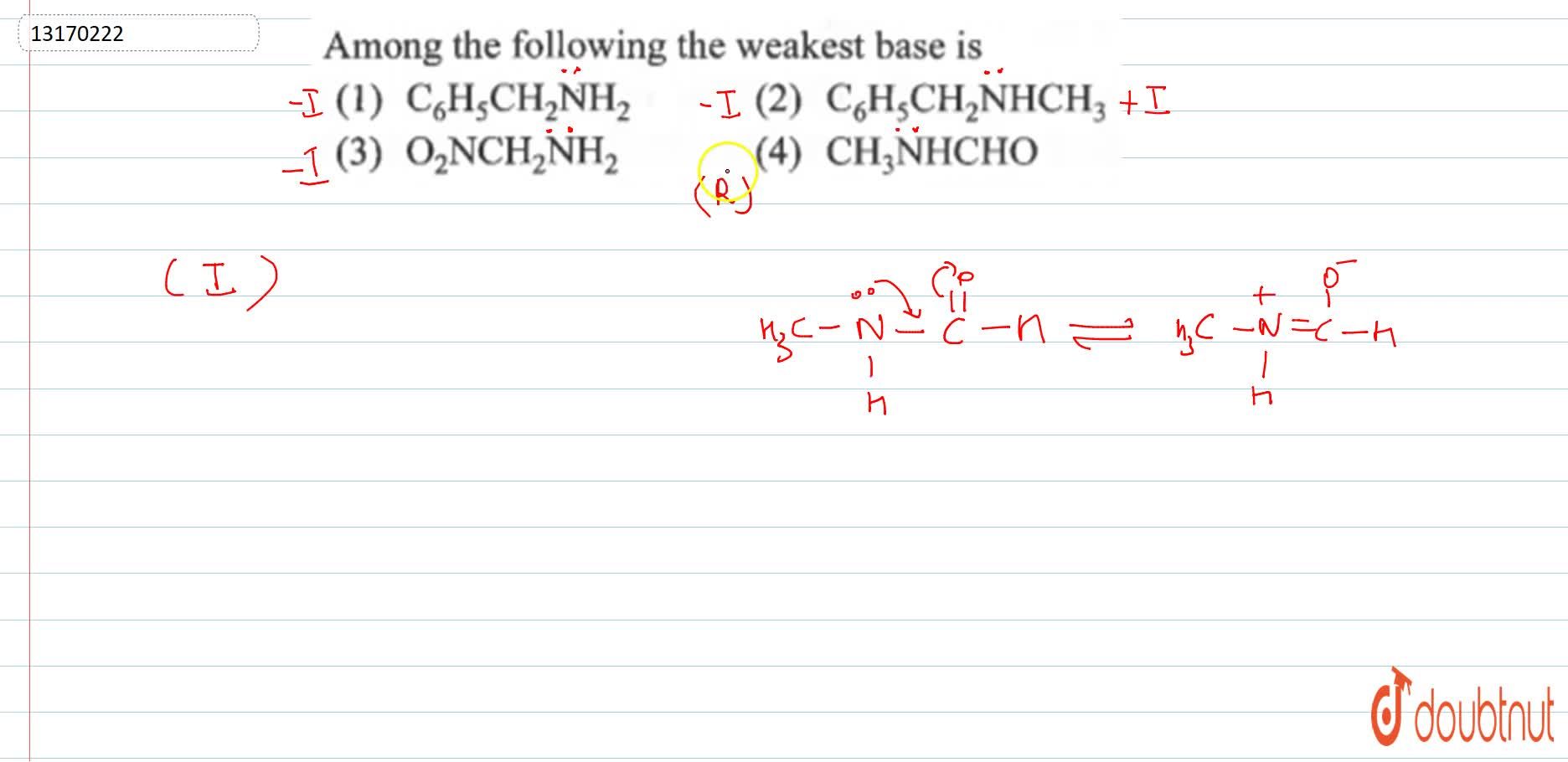 Source: doubtnut.com
Source: doubtnut.com
Which of the following is the weakest acid. (b) ammonia is the strongest reducing agent as well as the hydrides. The strongest acid among these will give weak base.
 Source: chegg.com
Source: chegg.com
(a) ammonia is the weakest reducing agent and the strongest base among group 15 hydrides. Taking it away from f is too hard. Which of the following is a weak base akoh bnaoh ccaoh2 dnh4oh.
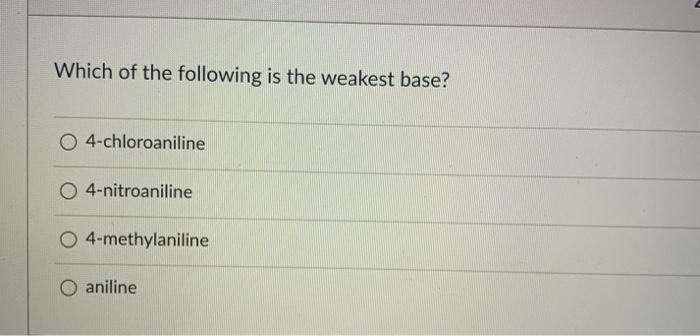
Therefore s the weakest base. So hf, is the strongest acid among the given acids. Ch3 ch3 о нас сн, ныс ch3 ch3 h5c о ныс ch2 the boat is the most stable conformation for this compound.
 Source: clutchprep.com
Source: clutchprep.com
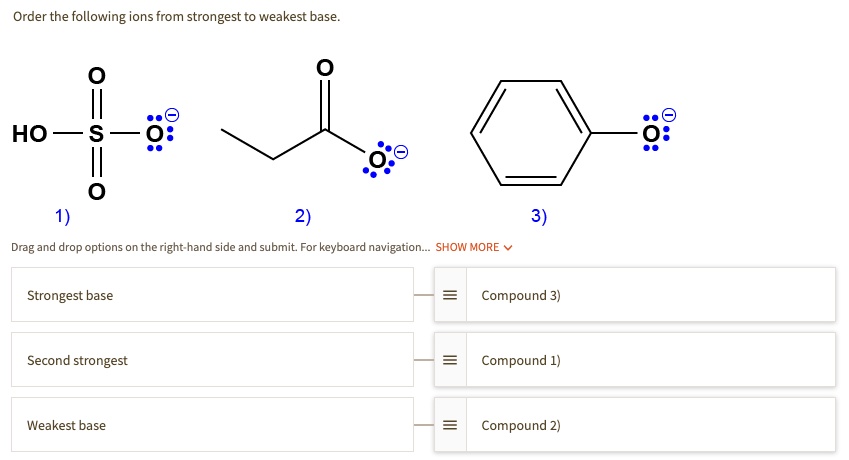
Benzenamine is the weakest base among the following since the delocalisation of the lone pair of electrons in it is not possible due to which the electron density on the molecule is fairly low and the compound acts as the weak base in the presence of the acid. If you got any suggestions to improve this website, please feel free to send message on pkmcqs. The following acids are listed in order of decreasing acid strength in water.
 Source: toppr.com
Source: toppr.com
Therefore, l i ( o h) is the weakest base. Here some of you may think that cyclohexylamine is showing strong basicity whether this was also attached with the ring. (c) ammonia is the weakest reducing agent as well as the weakest base among group 15 hydrides.

Hf > h2o > nh3 > ch4. Since hf is the strongest acid, it’s conjugate base will be the weakest base. Look at charges na, nh4 and k have +1 charge ca have +2 charge after dissociation.
 Source: youtube.com
Source: youtube.com
Zn(oh) 2 is a weak base. C ) the extent of dissociation of the dissolved acid or base. Which one of the following is the weakest acid?
 Source: brainly.in
Source: brainly.in
Which one of the following is the weakest acid? The appropriate method however is to add h to each base and check the strength of the acid. Which of the following is the weakest base?a.
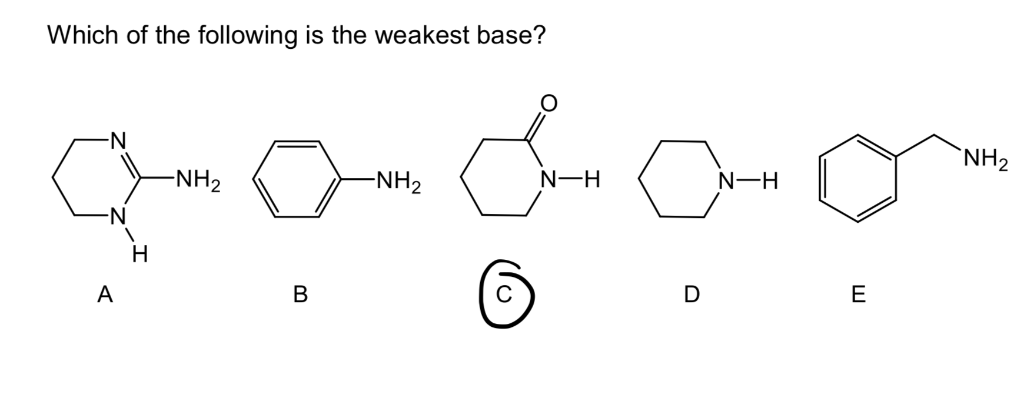 Source: chegg.com
Source: chegg.com
Which of the following is the weakest base? Hf > h2o > nh3 > ch4. (b) ammonia is the strongest reducing agent as well as the hydrides.
 Source: numerade.com
Source: numerade.com
Which of the following is the weakest acid. (a)ammonia is the weakest reducing agent and the strongest base among group 15 hydrides. Koh > naoh > ca(oh) 2 > zn(oh) 2.

Hence, zn(oh) 2 is the weakest base among the given options. Which of the following is the weakest base? So, from the above discussion, it is clear that aniline is the weakest base.
 Source: doubtnut.com
Source: doubtnut.com
(d) ammonia is the strongest reducing agent and the. The conjugate acid with the highest pka is methane ( c h x 4) it is the weakest acid of. (c) ammonia is the weakest reducing agent as well as the weakest base among group 15 hydrides.
 Source: youtube.com
Source: youtube.com
The reducing character of hydrides increases down the group due to decrease in bond dissociation enthalpy. The reducing character of hydrides increases down the group due to decrease in bond dissociation enthalpy. Chemistry mcqs, lioh , koh , naoh , rboh.
 Source: toppr.com
Source: toppr.com
(c) ammonia is the weakest reducing agent as well as the weakest base among group 15 hydrides. So it does not readily donate an electron pair making it the weakest lewis base. Also, it is the conjugate base of strong acid.
 Source: toppr.com
Source: toppr.com
The strongest acid among these will give weak base. So hf, is the strongest acid among the given acids. I�m fullstack web application developer.
 Source: chegg.com
Source: chegg.com
This means compound must have a strong lattice. Which of the following is the weakest base? (d)ammonia is the strongest reducing agent as well as the weakest base among group 15 hydrides.
 Source: numerade.com
Source: numerade.com
(b) ammonia is the strongest reducing agent as well as the hydrides. The decreasing order of basicity of given hydroxides are as follows: Look at charges na, nh4 and k have +1 charge ca have +2 charge after dissociation.
Also Read :





