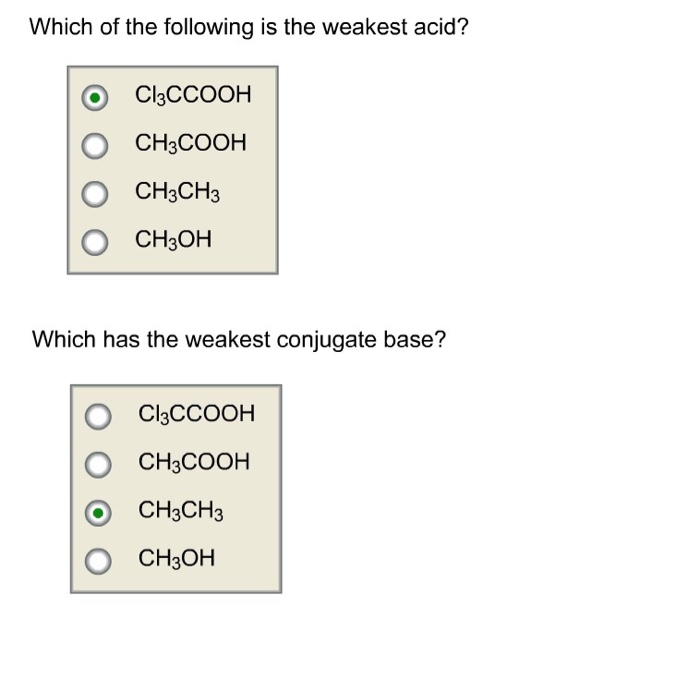
Cl3ccooh, ch3cooh, ch3ch3, or ch3oh which has the weakest conjugate base? ∴ phenol is the weakest acid.
Which Of The Following Is The Weakest Acid. Hence, option (a) is correct. Which one of the following is the weakest acid? Ch3oh c h 3 o h. A small ka value means little of the acid dissociates, then it is a weak acid.
 Chapter 17 Flashcards | Quizlet From quizlet.com
Chapter 17 Flashcards | Quizlet From quizlet.com
Related Post Chapter 17 Flashcards | Quizlet :
Cl3ccooh, ch3cooh, ch3ch3, or ch3oh which has the weakest conjugate base? So hf, is the strongest acid among the given acids. Answered sep 10, 2016 by sherrita. Fluorine being small in size overlaps better with 1s orbital of hydrogen leading to a strong bond.
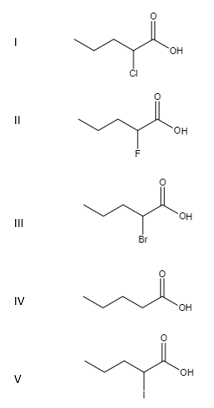
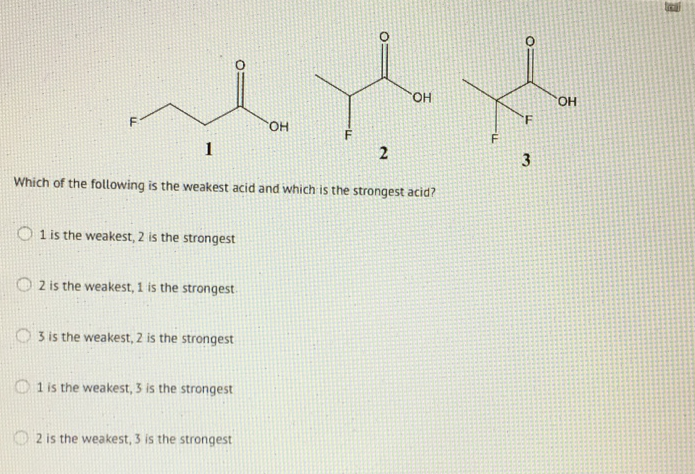
Which of the following is the weakest acid?
Which of the following is the weakest acid? Which of the following is the weakest acid? ∴ phenol is the weakest acid. This problem has been solved! Which of the following is the weakest acid? Which of the following is the weakest acid?
 Source: toppr.com
Source: toppr.com
A small ka value means little of the acid dissociates, then it is a weak acid. A high k a value indicates a lower ph for a solution. 15) of the following, _____ is a weak acid.
 Source: youtube.com
Source: youtube.com
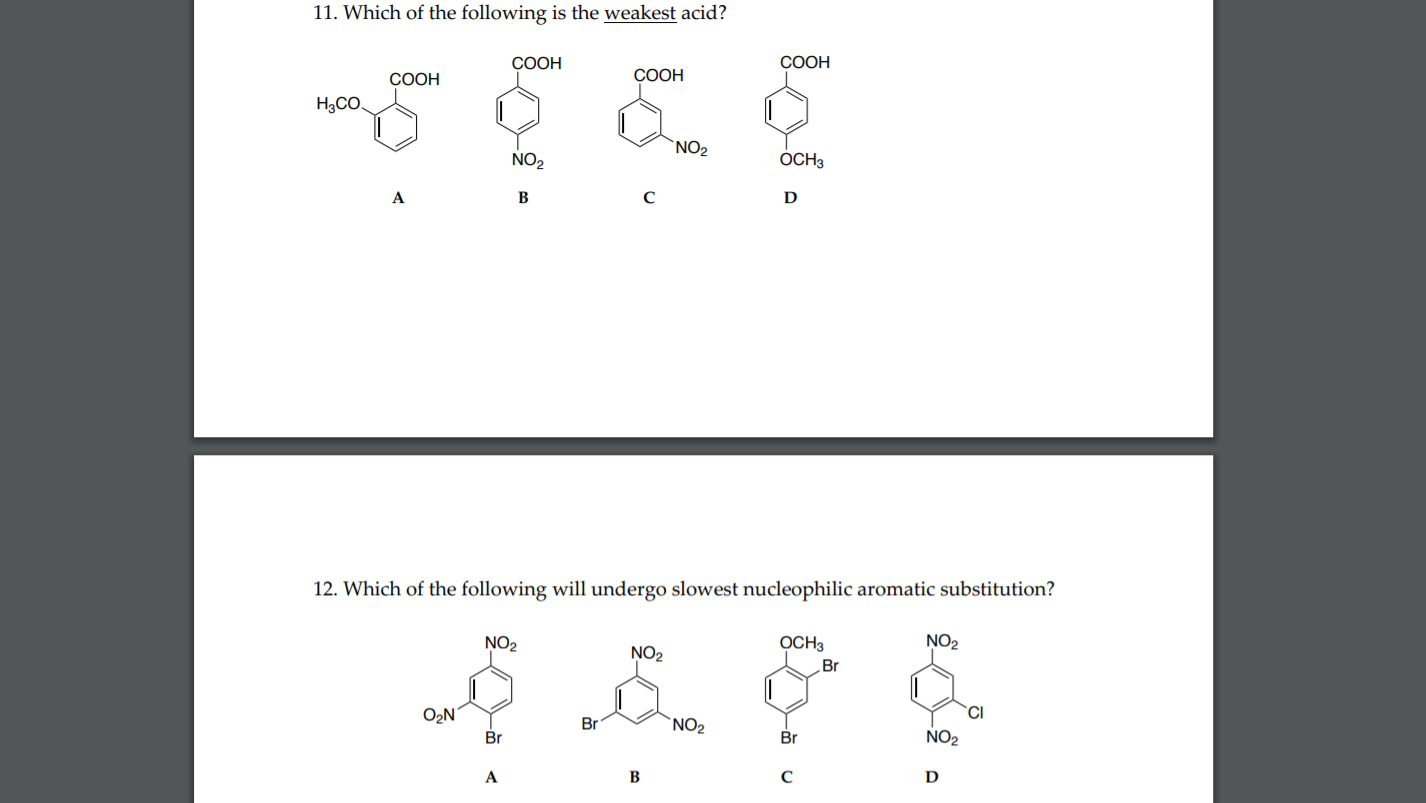
View solution > view more. Which one of the following is the weakest acid? Of the following, which is the weakest acid?
 Source: clutchprep.com
Source: clutchprep.com
View solution > view more. Ch3oh c h 3 o h. A high k a value indicates a lower ph for a solution.
 Source: study.com
Source: study.com
Answered sep 10, 2016 by sherrita. Which of the following acids will have the strongest conjugate base? This problem has been solved!
 Source: clutchprep.com
Source: clutchprep.com
Here bond strength overweighs the electronegativity of f. See the answer see the answer see the answer done loading. Ch3cooh c h 3 c o o h.
 Source: chegg.com
Source: chegg.com
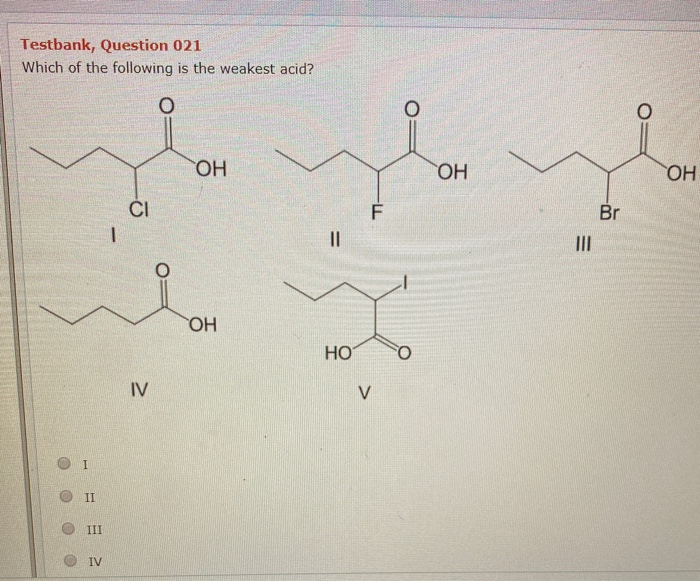
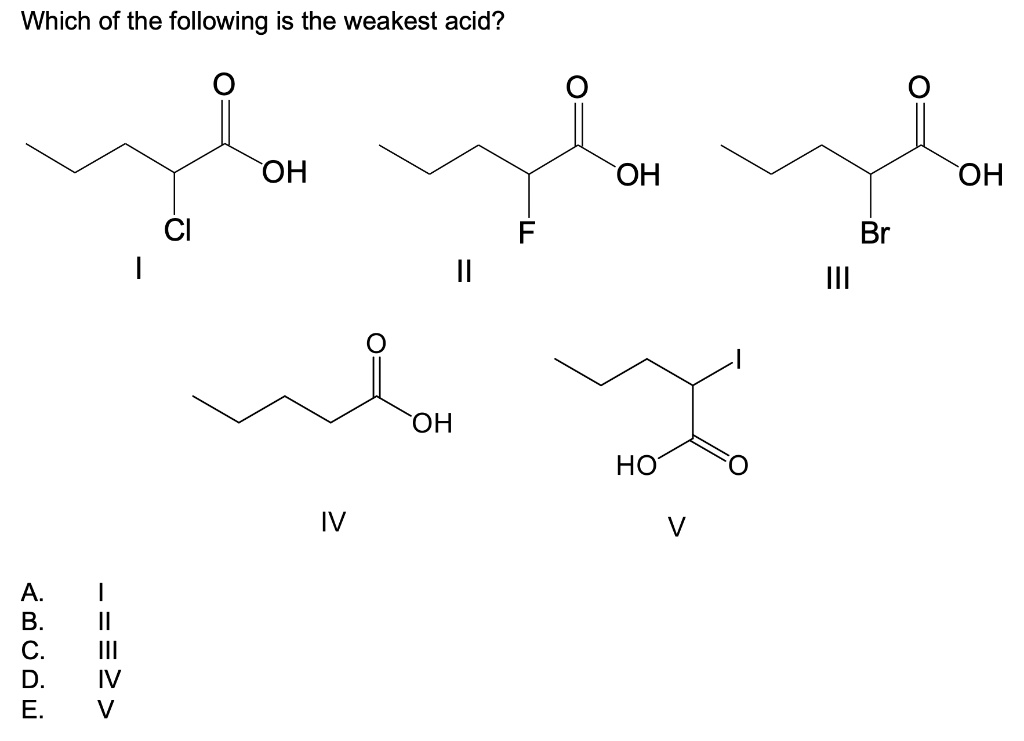
Which of the following is the weakest acid? Which of the following is the weakest acid? The acid strength increases as the experimental pka values decrease in the following order:
 Source: chegg.com
Source: chegg.com
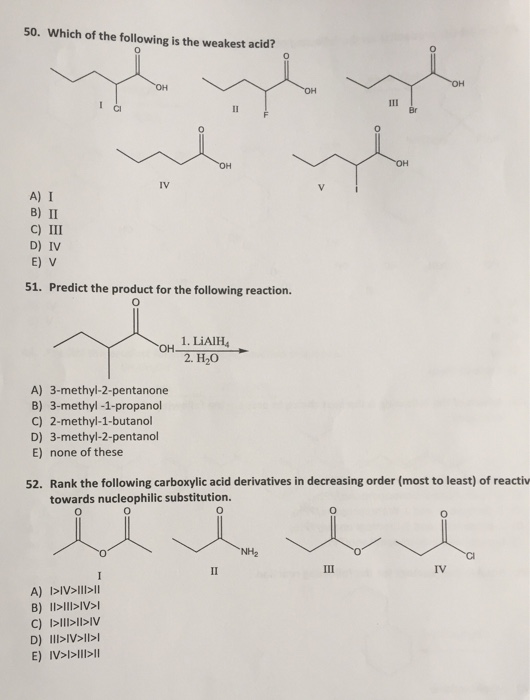
Which of the following is the weakest acid? All the best for your futurew endutrewd. Mathematically it is defined as the products and reactants both raised to their degree of coefficient, he higher ka is, the more easily the acid dissociates, and the stronger it is.
 Source: numerade.com
Source: numerade.com
Which one of the following is the weakest acid? Hcl, hbr, and hi are all strong acids, whereas hf is a weak acid. Ch3cooh c h 3 c o o h.
 Source: chegg.com
Source: chegg.com
Ch3cooh c h 3 c o o h. The acid strength increases as the experimental pka values decrease in the following order: Strong acids like hydrochloric acid, sulfuric acid, and nitric acid have very large.
 Source: transtutors.com
Source: transtutors.com
Cl3ccooh, ch3cooh, ch3ch3, or ch3oh which has the weakest conjugate base? Given the following equation, determine the amount of hcl (in grams) that is produced with 90.0 g fecl3. Class 5 the fish tale across the wall tenths and hundredths parts and whole can you see the pattern?
D) the conjugate base of a very weak acid is stronger than the conjugate base of a strong acid. Which one of the following is the weakest acid? The greater the value of k a, the more favoured the h + formation, which makes the solution more acidic.
 Source: chegg.com
Source: chegg.com
Phenol is the weakest acid. Here bond strength overweighs the electronegativity of f. So, hf is the weakest acid among them.
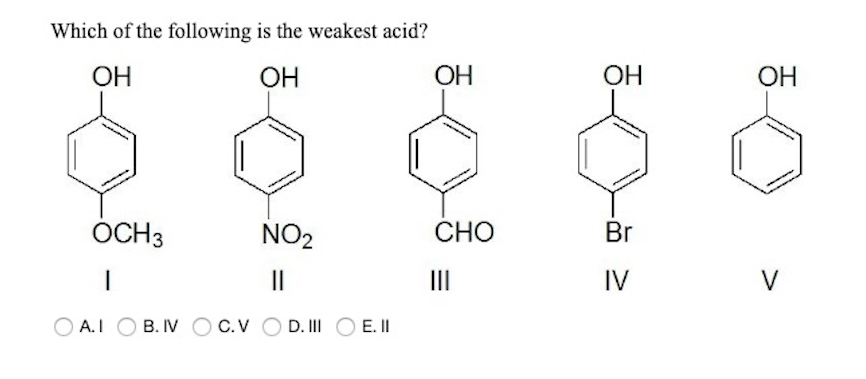 Source: chegg.com
Source: chegg.com
Phenol is the weakest acid. Ch3oh c h 3 o h. So, hf is the weakest acid among them.
 Source: oneclass.com
Source: oneclass.com
Hpo3 h2p04 the acid strength of all of these is the same. So, hf is the weakest acid among them. K a1 = 10 3.
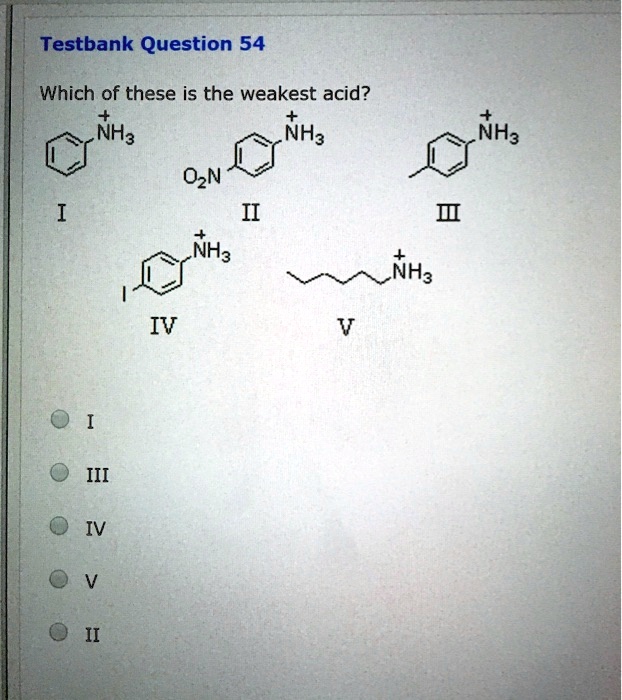 Source: numerade.com
Source: numerade.com
A large ka value indicates a strong acid because it means the acid is largely dissociated into its ions. The pka value of different acids is given : Fluorine being small in size overlaps better with 1s orbital of hydrogen leading to a strong bond.
 Source: quizlet.com
Source: quizlet.com
Which of the following is the weakest acid? A high k a value indicates a lower ph for a solution. Ch3cooh c h 3 c o o h.
 Source: toppr.com
Source: toppr.com
View solution > view more. The greater the value of k a, the more favoured the h + formation, which makes the solution more acidic. Which one of the following is the weakest acid?
 Source: toppr.com
Source: toppr.com
Which of the following is the weakest acid? Here bond strength overweighs the electronegativity of f. Which of the following is the weakest acid?
 Source: youtube.com
Source: youtube.com
In this problem that would be (d) hcn. Class 5 the fish tale across the wall tenths and hundredths parts and whole can you see the pattern? 16) which one of the following is the weakest acid?
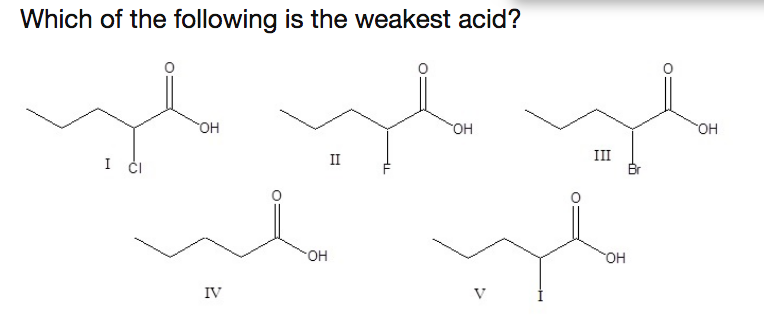 Source: chegg.com
Source: chegg.com
Neet question which of the following is the weakest acid? Which one of the following is the weakest acid? Hydrofluoric acid is the only weak acid in the choices.
Also Read :