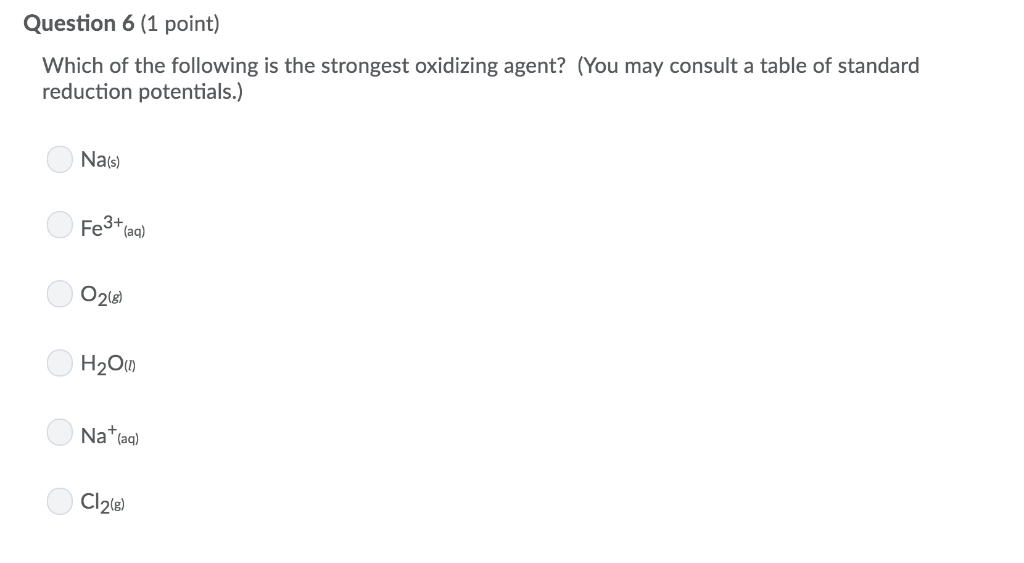
If all species are in their standard states, which of the following is the strongest oxidizing agent ? 400+ 8.8k+ which of the following is the most powerful oxidizing agent?
Which Of The Following Is The Strongest Oxidizing Agent. The strongest oxidizing agent among the following is: Most oxidising will also be hclo4 as the chlorine atom is in its highest oxidation state of +7 and thus undergoes reduction most readily to chloride anion. It is also known as an oxidizing agent to that element or compound that passes electronegative atoms to another substance. Which of the following statements about electrochemical cells is true?.
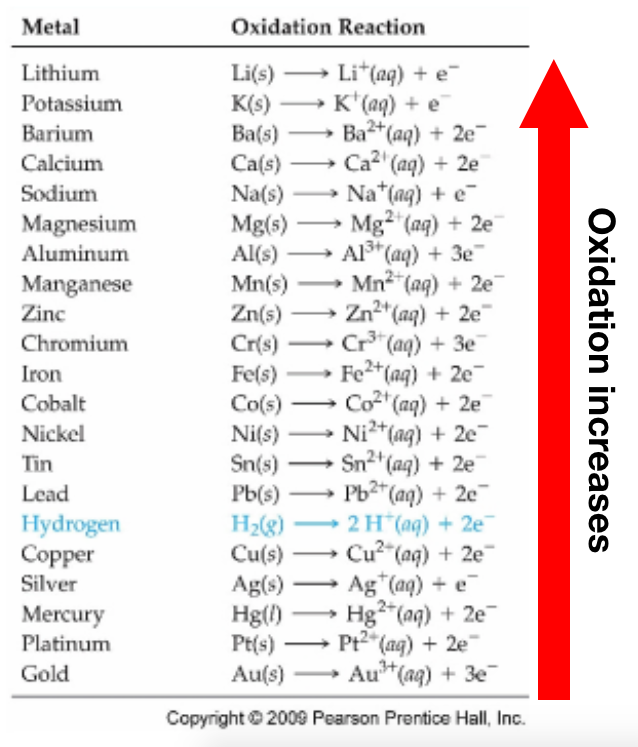 Which Substance Below Is The Strongest Red… | Clutch Prep From clutchprep.com
Which Substance Below Is The Strongest Red… | Clutch Prep From clutchprep.com
Related Post Which Substance Below Is The Strongest Red… | Clutch Prep :
Most oxidising will also be hclo4 as the chlorine atom is in its highest oxidation state of +7 and thus undergoes reduction most readily to chloride anion. Arrange the following in the order of the property indicated for each set : All halogens have a tendency to take up electrons and. (1) chlorine (2) iodine (3) fluorine (4) oxygen last answer :
As a result, the oxidizing agent is itself reduced by gaining electrons.
An example of a good oxidizing agent is an alkali metal, such as na. The substance with higher oxidation potential is the stronger oxidizing agent as higher the oxidation potential lower would be the gibbs� free energy. The higher the pull for electrons the stronger the oxidizing agent. The higher the pull for electrons the stronger the oxidizing agent. Are solved by group of students and teacher. If all species are in their standard states, which of the following is the strongest oxidizing agent ?

If all species are in their standard states, which of the following is the strongest oxidizing agent ? As a result, the oxidizing agent is itself reduced by gaining electrons. If all species are in their standard states, which of the following is the strongest oxidizing agent ?
 Source: oneclass.com
Source: oneclass.com
The strongest oxidizing agent among the following is: An oxidizing agent gains electrons. And here we can trade our be that mean reduction potential value reduction, but an in shell value.
 Source: chegg.com
Source: chegg.com
This discussion on among the following the strongest oxidizing agent is:? Is done on edurev study group by neet students. Fluorine is the most effective oxidizer, having the largest positive electrode potential.
 Source: pdfprof.com
Source: pdfprof.com
If all species are in their standard states, which of the following is the strongest oxidizing agent ? An oxidizing agent gains electrons. F 2 is the best oxidizing agent of the periodic table.
 Source: clutchprep.com
Source: clutchprep.com
Strong oxidizers are capable of forming explosive mixtures when mixed with combustible, organic or easily oxidized materials. All halogens have a tendency to take up electrons and. Fluorine is said to be the strongest elemental oxidizing agent due to its highest electronegativity, as discussed earlier.
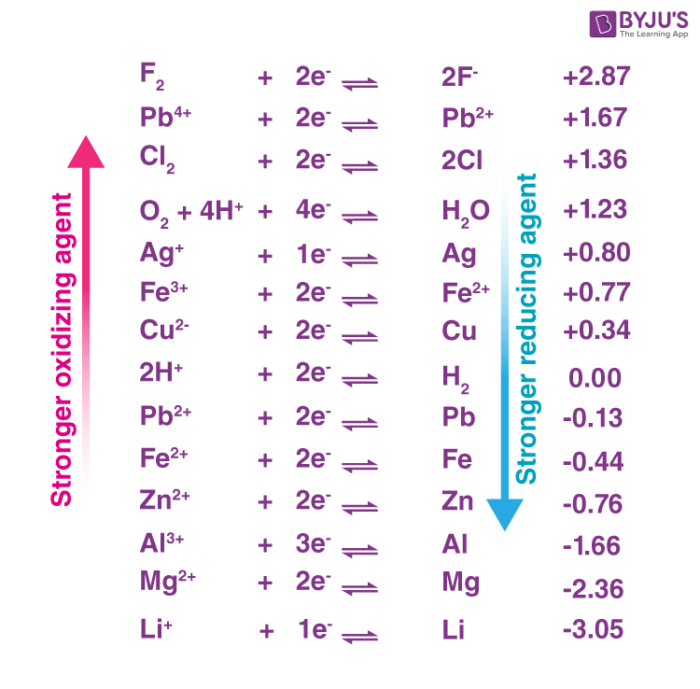 Source: byjus.com
Source: byjus.com
400+ 8.8k+ which of the following is the most powerful oxidizing agent? This could be because fluorine is the most electronegative element in the present periodic table, and hence has the highest attractive force on electrons of all the elements. As a result, the oxidizing agent is itself reduced by gaining electrons.
 Source: youtube.com
Source: youtube.com
When studying chemical reactions, all the substances involved and the processes that occur in them. The most positive oxidation potential would be +1.66vand al (not al3+) is the reactant that is getting oxidized and is the strongest reducing agent (choice b). The substance with higher oxidation potential is the stronger oxidizing agent as higher the oxidation potential lower would be the gibbs� free energy.

F 2 is the best oxidizing agent of the periodic table. Which of the following is the strongest reducing agent in acidic solution: So so based on the reduction potential values.
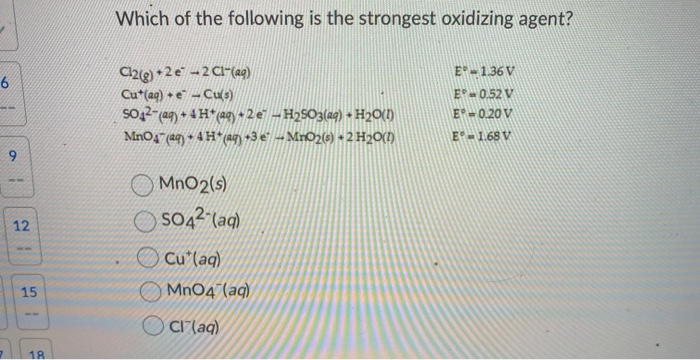 Source: chegg.com
Source: chegg.com
So c l minus is equal to 1.36 fold. So the element with the highest electronegativity is the strongest oxidizing agent. This could be because fluorine is the most electronegative element in the present periodic table, and hence has the highest attractive force on electrons of all the elements.

What are some strong oxidizing agents? The standard electrode potential of a substance gives an indication of how strong. Apart from being the largest neet community, edurev has the largest solved question bank for neet.
 Source: toppr.com
Source: toppr.com
And here we can trade our be that mean reduction potential value reduction, but an in shell value. For f 2 , e o (rp) is minimum. Which of the following statements about electrochemical cells is true?.
 Source: commons.wikimedia.org
Source: commons.wikimedia.org
Which of the following is the strongest reducing agent in acidic solution: The substance with higher oxidation potential is the stronger oxidizing agent as higher the oxidation potential lower would be the gibbs� free energy. Is done on edurev study group by neet students.
 Source: moviecultists.com
Source: moviecultists.com
Fluorine, having the largest positive value of electrode potential, is the strongest. The most positive oxidation potential would be +1.66vand al (not al3+) is the reactant that is getting oxidized and is the strongest reducing agent (choice b). The species at the top left have the greatest potential to be reduced, so they are the strongest oxidizing agents.
 Source: youtube.com
Source: youtube.com
Fluorine is said to be the strongest elemental oxidizing agent due to its highest electronegativity, as discussed earlier. So the element with the highest electronegativity is the strongest oxidizing agent. Common oxidizing agents are oxygen, hydrogen peroxide and the halogens.
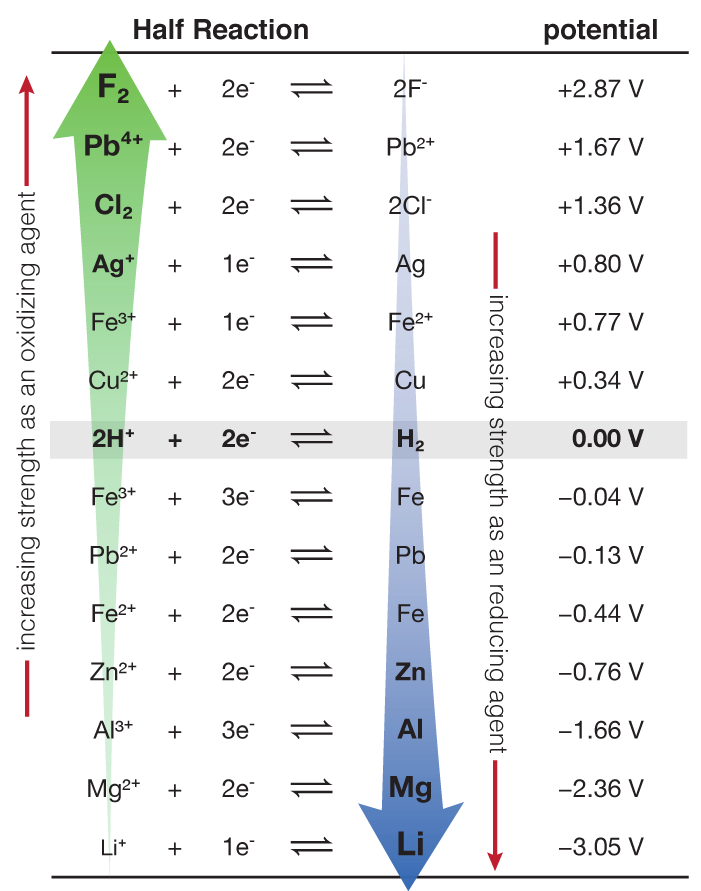 Source: ch302.cm.utexas.edu
Source: ch302.cm.utexas.edu
As a result, the oxidizing agent is itself reduced by gaining electrons. If all species are in their standard states, which of the following is the strongest oxidizing agent ? The halogen which has a higher value of standard reduction potential will be the strongest oxidizing agent.
 Source: clutchprep.com
Source: clutchprep.com
Most oxidising will also be hclo4 as the chlorine atom is in its highest oxidation state of +7 and thus undergoes reduction most readily to chloride anion. Arrange the following in the order of the property indicated for each set : The strongest oxidizing agent is fluorine with the largest positive number for.
 Source: doubtnut.com
Source: doubtnut.com
Is done on edurev study group by neet students. What are some strong oxidizing agents? So the element with the highest electronegativity is the strongest oxidizing agent.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
Which of the following statements about electrochemical cells is true?. Fluorine, having the largest positive value of electrode potential, is the strongest. The questions and answers of among the following the strongest oxidizing agent is:?
 Source: chegg.com
Source: chegg.com
Is done on edurev study group by neet students. Fluorine, having the largest positive value of electrode potential, is the strongest. If all species are in their standard states, which of the following is the strongest oxidizing agent ?
Also Read :





