Which one of the following is the strongest base in aqueous medium : This is the best answer based on feedback and ratings.
Which Of The Following Is The Strongest Nucleophile. Oc and d are equally strong b a and d are equally strong b and d are equally strong a and b are equally strong ос a and care equally strong b and care equally strong a. There are two factors we must look at to assess the strength of a nucleophile: The strongest nucleophile among the following is. Molecule 1 and 2 are more.
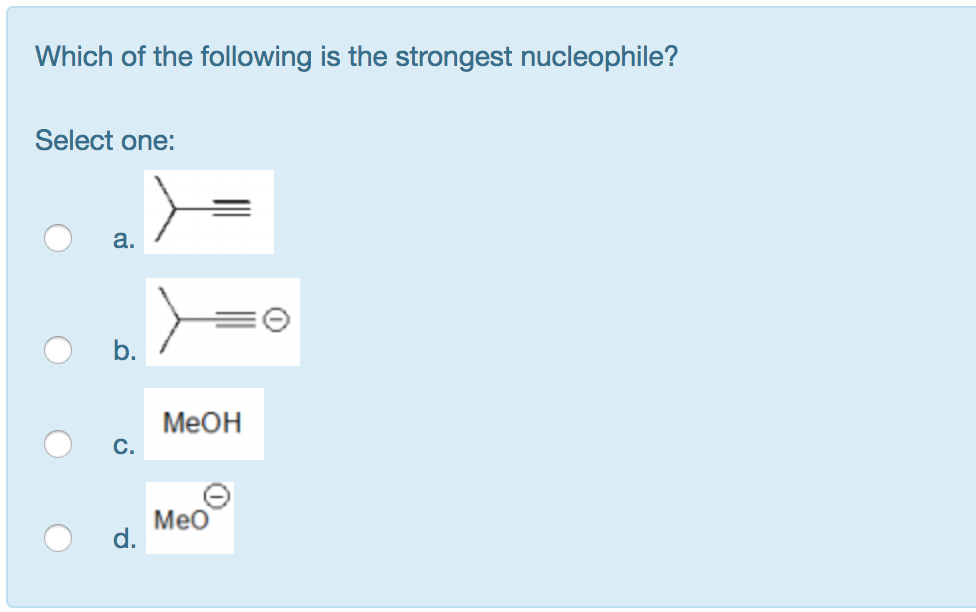 Solved Which Of The Following Is The Strongest Nucleophile? | Chegg.com From chegg.com
Solved Which Of The Following Is The Strongest Nucleophile? | Chegg.com From chegg.com
Related Post Solved Which Of The Following Is The Strongest Nucleophile? | Chegg.com :
A negative charge will always be a stronger nucleophile than its neutral counterpart. The strongest nucleophile among the following is. Oc and d are equally strong b a and d are equally strong b and d are equally strong a and b are equally strong ос a and care equally strong b and care equally strong a. View solution > for the following reaction,.
More is the electron density, more will be the nucleophilicity or donation power.
In an aprotic solvent, h2o is the strongest nucleophile. What is a good electrophile? Complete step by step solution: Substrate reagent product reagents are of two types: Which one of the following is the strongest base in aqueous medium : Which is the weakest nucleophile?
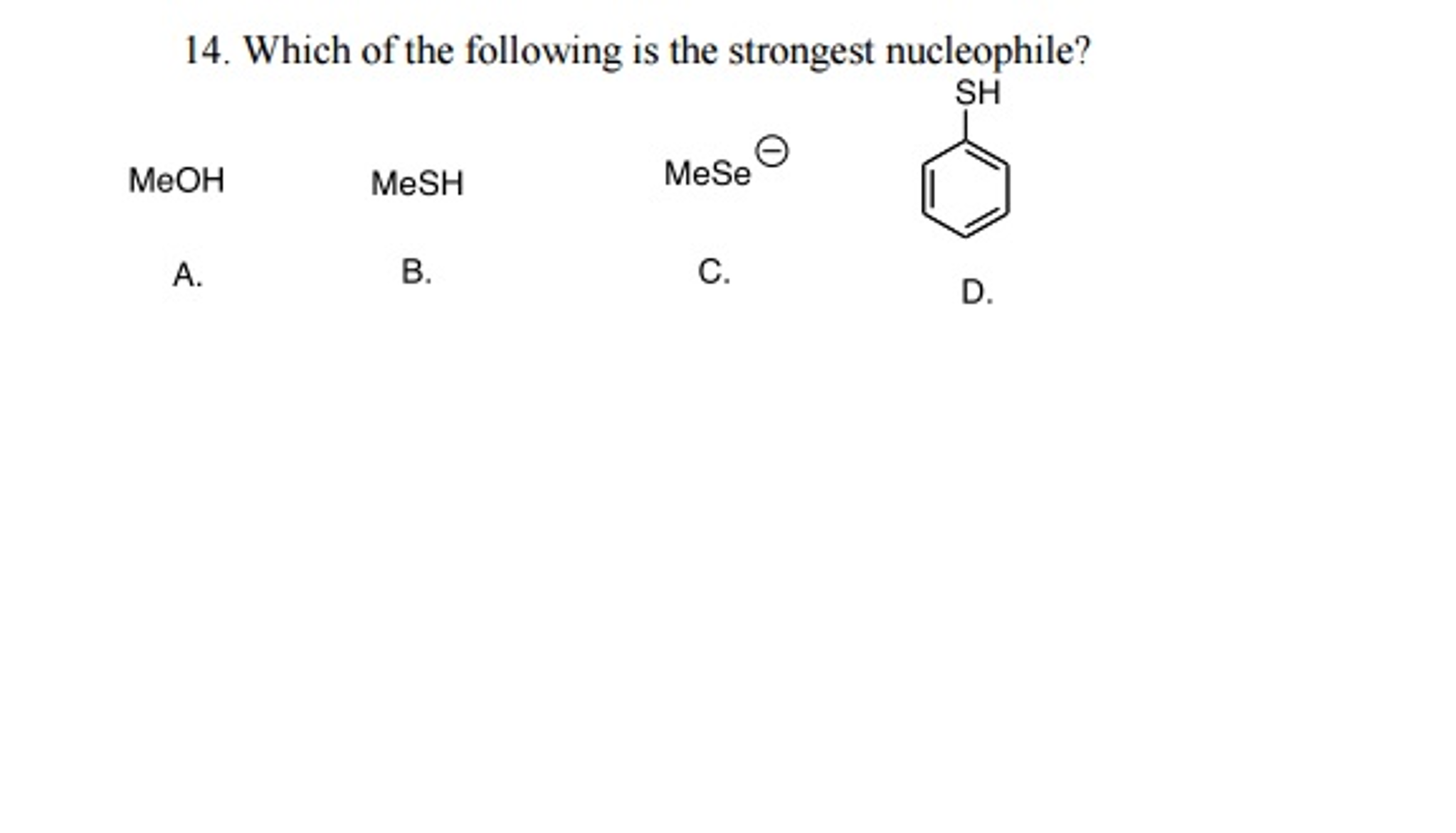 Source: chegg.com
Source: chegg.com
This means in a protic solvent, the strongest nucleophile among the given is c. 9.56 for each of the following pairs of species, which is the stronger nucleophile in acetone? Having the question is which of the following is their strong western if you file.
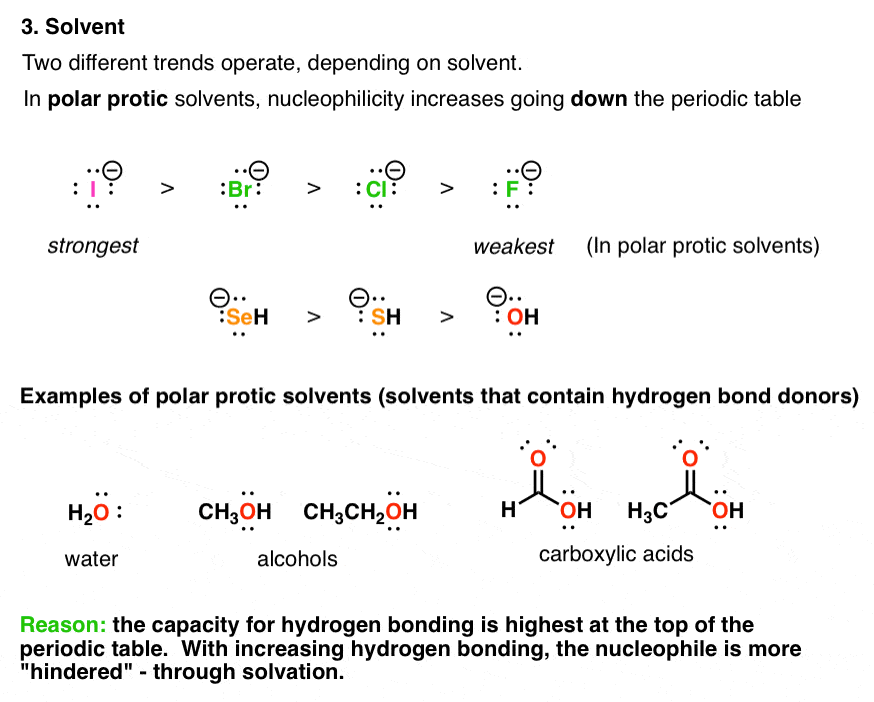 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
This question can also be solved using p k a values. This is because it can react at more sites and will not be. The strongest nucleophile among the following is.

Basicity and nucleophilicity have opposite size. Molecule 1 and 2 are more. Oc and d are equally strong b a and d are equally strong b and d are equally strong a and b are equally strong ос a and care equally strong b and care equally strong a.
 Source: quizlet.com
Source: quizlet.com
Basicity and nucleophilicity have opposite size. The strongest nucleophile among the following is. C 2 h 5 o ¯.
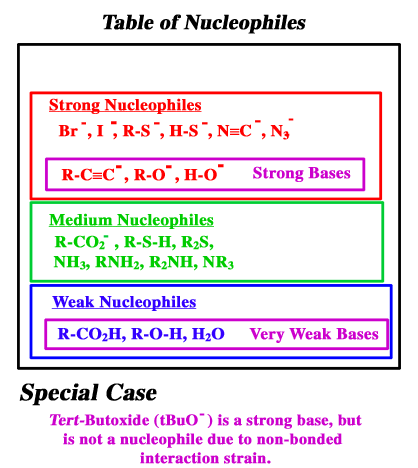 Source: iverson.cm.utexas.edu
Source: iverson.cm.utexas.edu
View solution > for the following reaction,. The bulkier the base, the more basic and less nucleophilic it is. C n − > i − > c 6 h 5 o − > o h − > b r − > c l −.
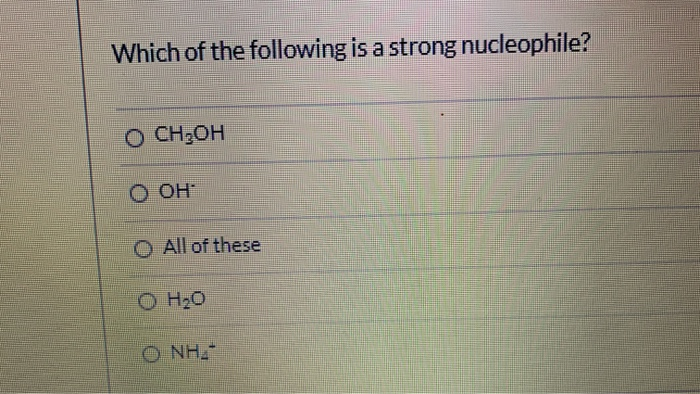 Source: chegg.com
Source: chegg.com
Basicity and nucleophilicity have opposite size. Which of the following is the strongest nucleophile? As c h 3oh c h 3 o h is the weakest acid while c h 3+ oh 2.
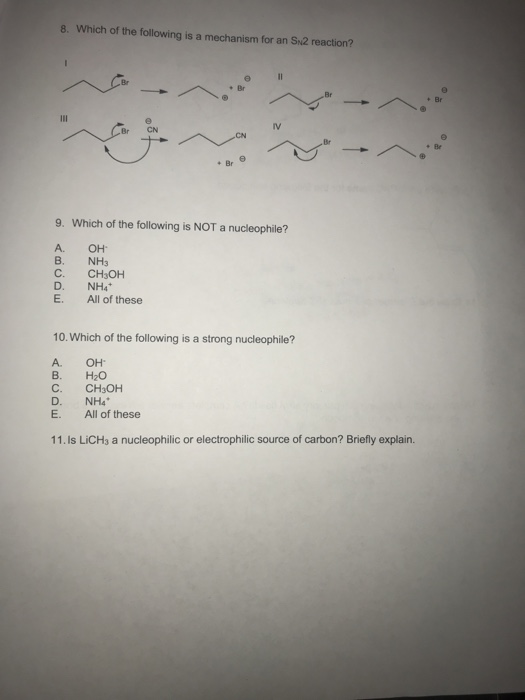 Source: chegg.com
Source: chegg.com
That implies, ethoxide ion is the strongest base because the conjugate base of ethanol will be a strong acid. Which of the following is the strongest nucleophile ? Oc and d are equally strong b a and d are equally strong b and d are equally strong a and b are equally strong ос a and care equally strong b and care equally strong a.
 Source: chegg.com
Source: chegg.com
The small size causes its charge to be extremely condensed, and in organic chemistry, the lack of ability to spread out the. For teachers for schools for working scholars. C n − > i − > c 6 h 5 o − > o h − > b r − > c l −.
 Source: chegg.com
Source: chegg.com
There are two factors we must look at to assess the strength of a nucleophile: View solution > for the following reaction,. Basicity and nucleophilicity have opposite size.
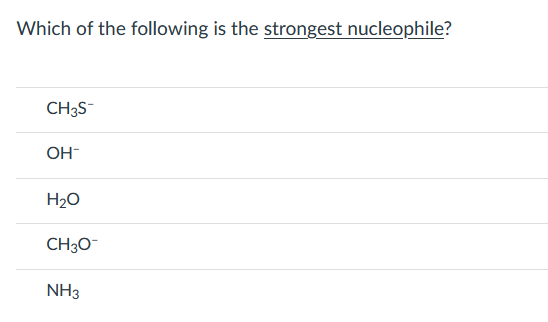 Source: bartleby.com
Source: bartleby.com
Which of the following is the strongest nucleophile ? As c h 3oh c h 3 o h is the weakest acid while c h 3+ oh 2. Among the following the strongest nucleophile is.
![Solved] Which Of The Following Is The Strongest Nucleophile? (A) (B) Hn (C) Bo (D) Ho Which Substrate Will Undergo The Fastest S� Reaction? (A) (B )… | Course Hero](https://www.coursehero.com/qa/attachment/12823244/ “Solved] Which Of The Following Is The Strongest Nucleophile? (A) (B) Hn (C) Bo (D) Ho Which Substrate Will Undergo The Fastest S� Reaction? (A) (B )… | Course Hero”) Source: coursehero.com
Which of the following is the strongest nucleophile? View solution > for the following reaction,. For teachers for schools for working scholars.
 Source: numerade.com
Source: numerade.com
Therefore, c 2 h 5 o − is the strongest nucleophile. Ammonia is a stronger nucleophile than water because nitrogen is less electronegative than oxygen. The strongest nucleophile among the following is.
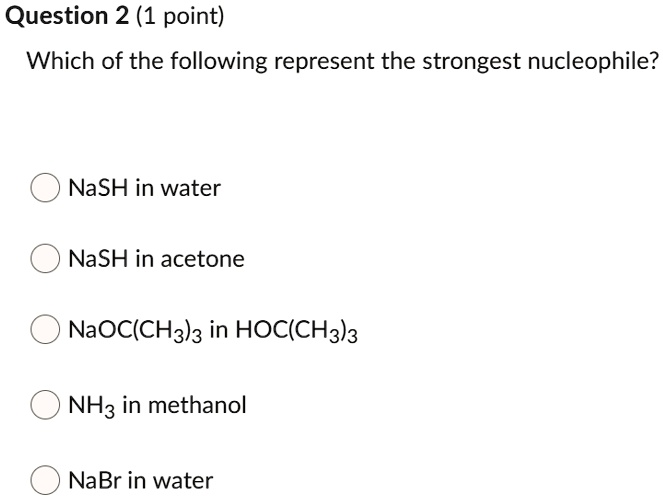 Source: numerade.com
Source: numerade.com
Which of the following is the strongest nucleophile? The polarizability and the stability. As c h 3oh c h 3 o h is the weakest acid while c h 3+ oh 2.
 Source: chegg.com
Source: chegg.com
So we are going to discuss which of the following given is that the strongest revival negative publicity increase as a negative charge containing element has the less electron negatory. The strength of nucleophile depends upon the nature of alkyl group r on which nucleophile has to attack and also on the nature of solvent. Thus stronger will be the nucleophile.
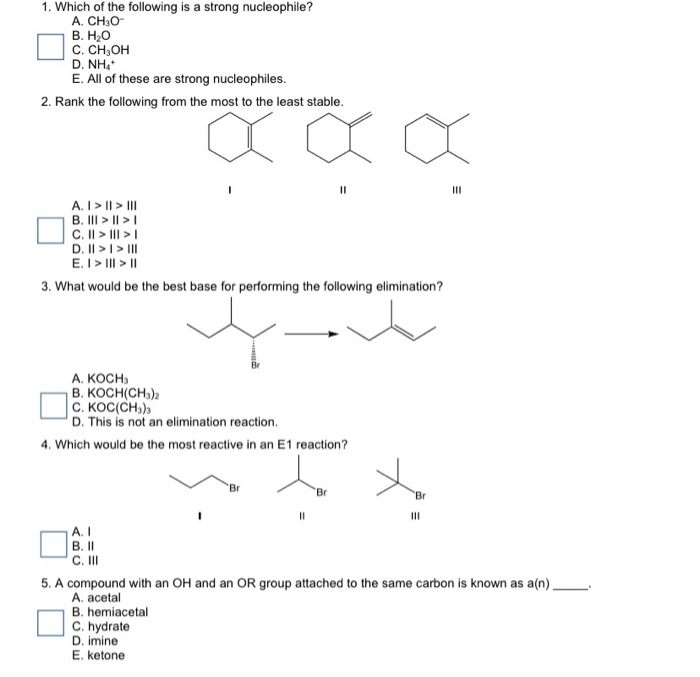 Source: chegg.com
Source: chegg.com
The small size causes its charge to be extremely condensed, and in organic chemistry, the lack of ability to spread out the. The bulkier the base, the more basic and less nucleophilic it is. The order of strength of nucleophiles follows the order :
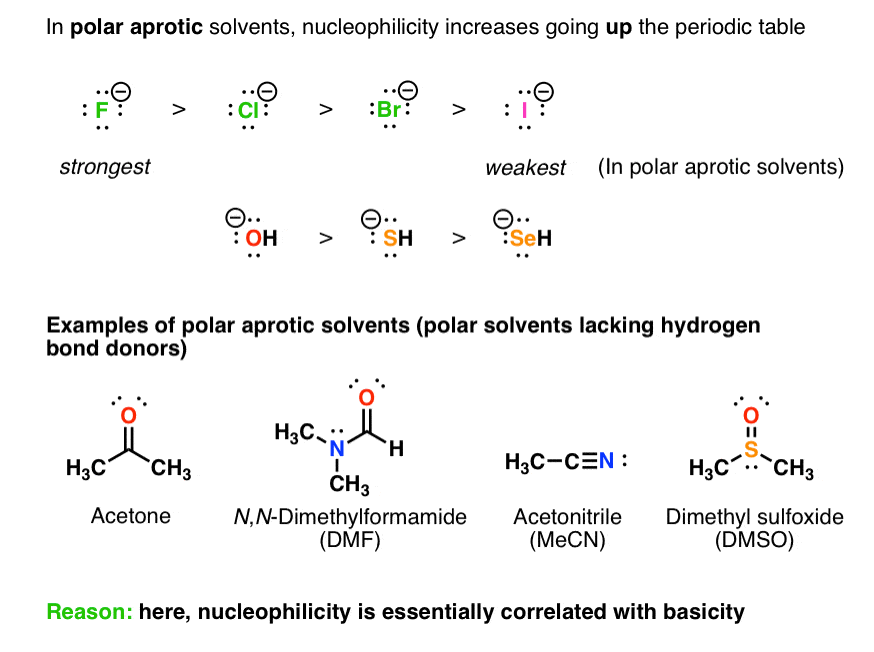 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
As c h 3oh c h 3 o h is the weakest acid while c h 3+ oh 2. Therefore, c 2 h 5 o − is the strongest nucleophile. Basicity and nucleophilicity have opposite size.
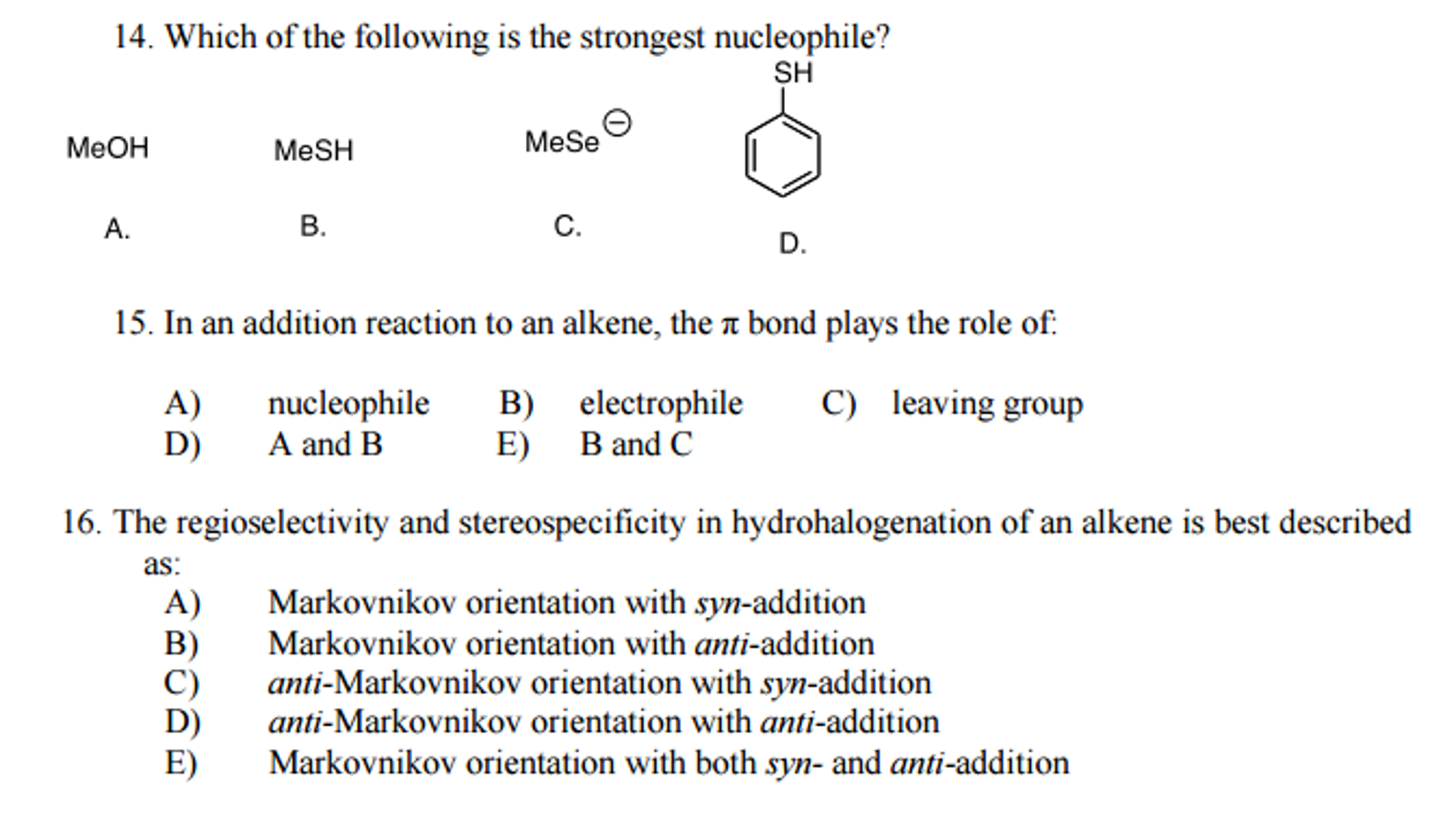 Source: chegg.com
Source: chegg.com
There are two factors we must look at to assess the strength of a nucleophile: In an aprotic solvent, h2o is the strongest nucleophile. Recall that the rules for nucleophile strength are:
 Source: bartleby.com
Source: bartleby.com
This means in a protic solvent, the strongest nucleophile among the given is c. Higher the p k a, stronger is the base and weaker is the acid. The strength of nucleophile depends upon the nature of alkyl group r on which nucleophile has to attack and also on the nature of solvent.
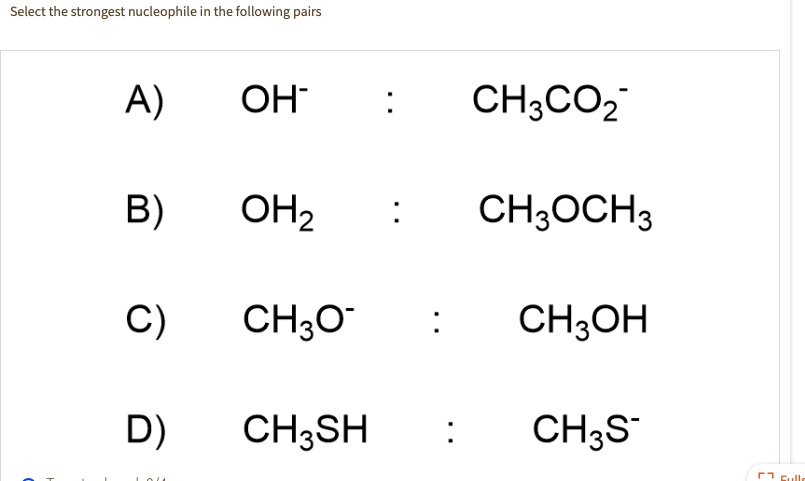 Source: numerade.com
Source: numerade.com
Which of the following is the strongest nucleophile ? Which of the following is strongest nucleophile? More is the electron density, more will be the nucleophilicity or donation power.
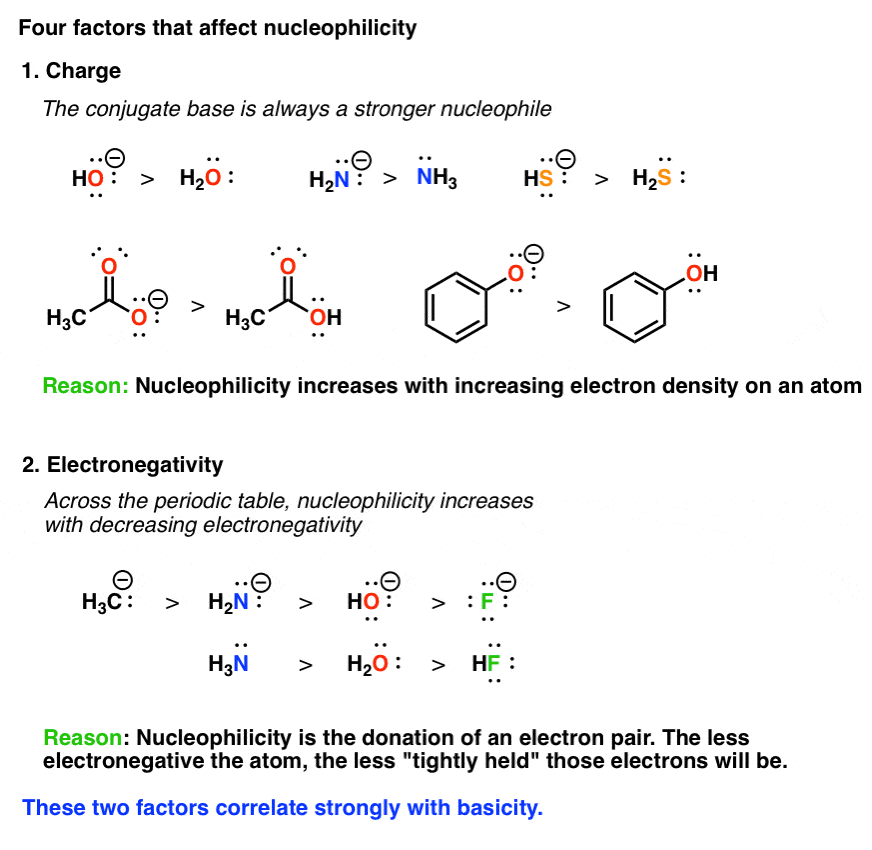 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
Basicity and nucleophilicity have opposite size. Substrate reagent product reagents are of two types: Oc and d are equally strong b a and d are equally strong b and d are equally strong a and b are equally strong ос a and care equally strong b and care equally strong a.
Also Read :