I'd consider kmno4 to be a strong oxidizing agent under any conditions. Ch 3 cooh ____ 18.
Which Of The Following Is The Strongest Acid. But ek says d is the answer since h3po4 loses protons. Which one of the following is a strong acid? Fch2cooh > clch2cooh > brch2cooh > ich2cooh. This bond decreases the boron atom�s electron deficiency, and its lewis acid character diminishes.
 Oneclass: Which Of The Following Is The Strongest Acid? Which Of The Following Is The Strongest … From oneclass.com
Oneclass: Which Of The Following Is The Strongest Acid? Which Of The Following Is The Strongest … From oneclass.com
Related Post Oneclass: Which Of The Following Is The Strongest Acid? Which Of The Following Is The Strongest … :
Identify the strongest acid in each of the following sets. Thus [b{i_3}]behaves as a lewis acid which is. This bond decreases the boron atom�s electron deficiency, and its lewis acid character diminishes. Which response includes all the acids listed below that are strong acids, and no weak acids?
Which of the following is the strongest acid?
In m − c h 3 o c 6 h 4 c o o h and p − c h 3 o c 6 h 4 c o o h, the − o c h 3 group� being electron donating makes benzoic acid weaker. Which of the following is the strongest acid? Nf 3 is a weaker base than : Which among the following is the strongest acid ? Chloric acid, hydrobromic acid, hydrochloric acid, hydroiodic acid, nitric acid, perchloric acid, and sulfuric acid. Which response includes all the acids listed below that are strong acids, and no weak acids?
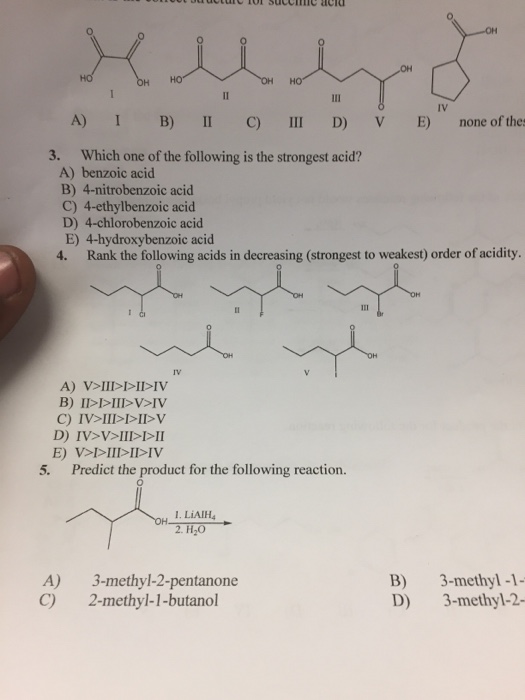
H n o3,pka = − 1.4. Fluoroantimonic acid is the world’s strongest acid, proudly standing on the pedestal slightly above carborane. A) ch4 b) nh3 c) h2o d) hf 2.
 Source: toppr.com
Source: toppr.com
C 6 h 5 c o o h is the strongest acid. A) ch4 b) nh3 c) h2o d) hf 2. But ek says d is the answer since h3po4 loses protons.
 Source: clutchprep.com
Source: clutchprep.com
C 6 h 5 c o o h is the strongest acid. This bond decreases the boron atom�s electron deficiency, and its lewis acid character diminishes. As physical scientists, however, we should look at actual measurements, namely the pka values of each acid in water.
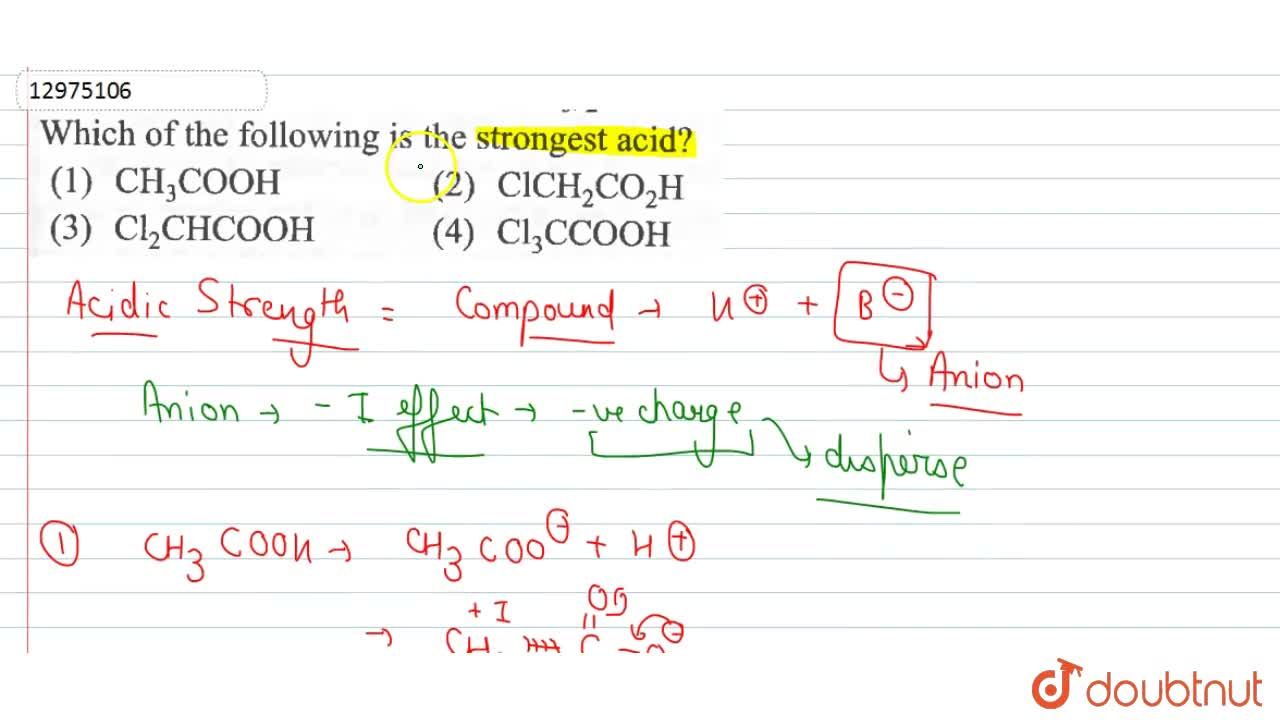 Source: doubtnut.com
Source: doubtnut.com
Being part of the list of strong acids doesn’t give any indication of how dangerous or damaging an acid is though. Asked aug 4, 2021 in chemistry by kanishk01 (45.9k points) edited aug 10, 2021 by faiz. Nh 4 + ____ 19.
 Source: brainly.in
Source: brainly.in
Which of the following is the strongest acid? Identify the strongest intermolecular forces present in each of the following: The strong acids and bases are simply those that completely dissociate in water.
 Source: youtube.com
Source: youtube.com
There are 7 strong acids: The strongest acid is perchloric acid on the left, and the weakest is hypochlorous acid on the far right. Fluoroantimonic acid is the world’s strongest acid, proudly standing on the pedestal slightly above carborane.
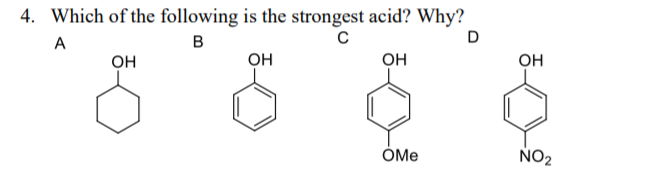 Source: bartleby.com
Source: bartleby.com
A) i b) ii c) iii d) iv e) v 11) explain why : A certain substance is classified as a lewis acid. Nh 4 + ____ 19.
 Source: techwhiff.com
Source: techwhiff.com
A) h2co3 b) h2so3 c) h2so4 d) h3po4 e) ch3cooh free expert solution show answer answer: Only salts of (weak acid + strong base) and (strong acid + weak base) get hydrolysed (i.e., show alkalinity or acidity in water). There are 7 strong acids:
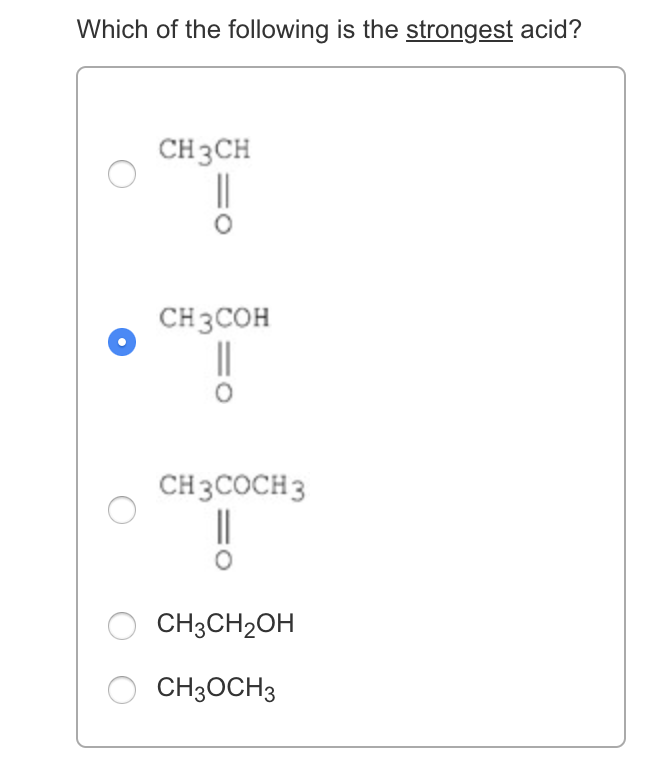 Source: chegg.com
Source: chegg.com
Asked aug 4, 2021 in chemistry by kanishk01 (45.9k points) edited aug 10, 2021 by faiz. H 2 so 4 b. But ek says d is the answer since h3po4 loses protons.
 Source: toppr.com
Source: toppr.com
Nf 3 is a weaker base than : Identify the strongest acid in each of the following sets. Thus, c l o − will be strongest base and so its conjugate acid hclo will be the weakest acid.
![Solved] 17. Which Of The Following Is The Strongest Acid? A. Hf B. Hbr C. Hci D. Hi 18. Which Of The Following Is The Strongest… | Course Hero](https://www.coursehero.com/qa/attachment/14950263/ “Solved] 17. Which Of The Following Is The Strongest Acid? A. Hf B. Hbr C. Hci D. Hi 18. Which Of The Following Is The Strongest… | Course Hero”) Source: coursehero.com
Fch2cooh > clch2cooh > brch2cooh > ich2cooh. Being part of the list of strong acids doesn’t give any indication of how dangerous or damaging an acid is though. Nh 3 12) the pka of ch 3 cooh is 4.8.
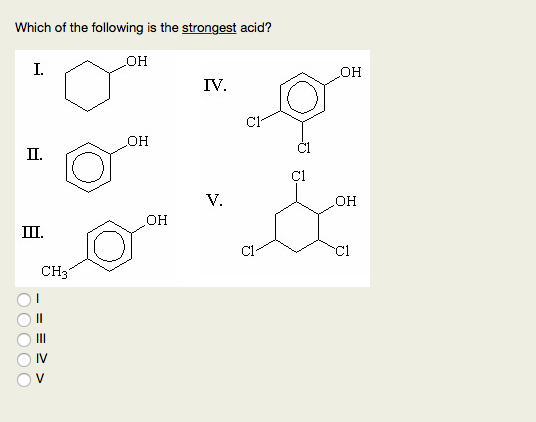 Source: chegg.com
Source: chegg.com
Identify the strongest intermolecular forces present in each of the following: H n o3,pka = − 1.4. Identify the strongest intermolecular forces present in each of the following:
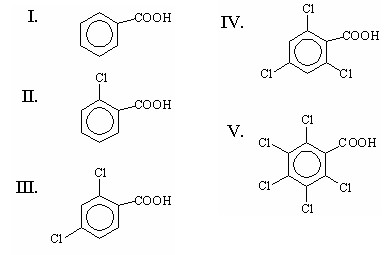 Source: chegg.com
Source: chegg.com
The strongest acid is perchloric acid on the left, and the weakest is hypochlorous acid on the far right. Which is a stronger acid? H 2so4,pka1 = − 3,pka2 = 1.99.
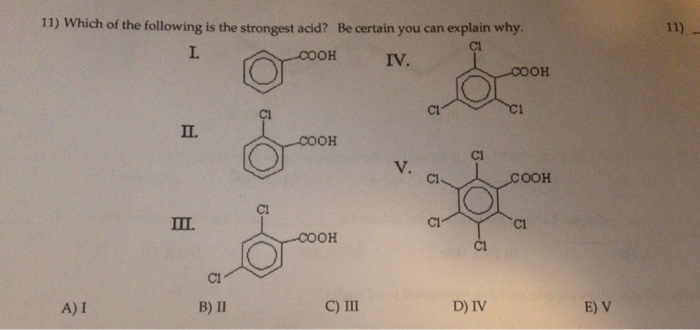
Which among the following is the strongest acid ? But let�s look at the following reactions: Which of the following is the strongest acid?
 Source: youtube.com
Source: youtube.com
Ch3ch2ch3 c6h5nh2 hf so2 ch3ch2oh nf3 ch3cl chemistry a buffer is prepared using acetic acid, ch3cooh, (a weak acid, pka = 4.75) and sodium acetate, ch3coona (which provides acetate ions, the conjugate base), according to the following proportions: Hence, the acid is strong. Nh 4 + ____ 19.
 Source: oneclass.com
Source: oneclass.com
Notice that the only difference between these acids is the number of oxygens bonded to chlorine. Fch2cooh is the strongest acid out of all. Which of the following is the weakest acid?
 Source: oneclass.com
Source: oneclass.com
H 2 nnh 2 e. Which of the following is the strongest acid? Which of the following statements explain why hbr is a stronger acid than hf?
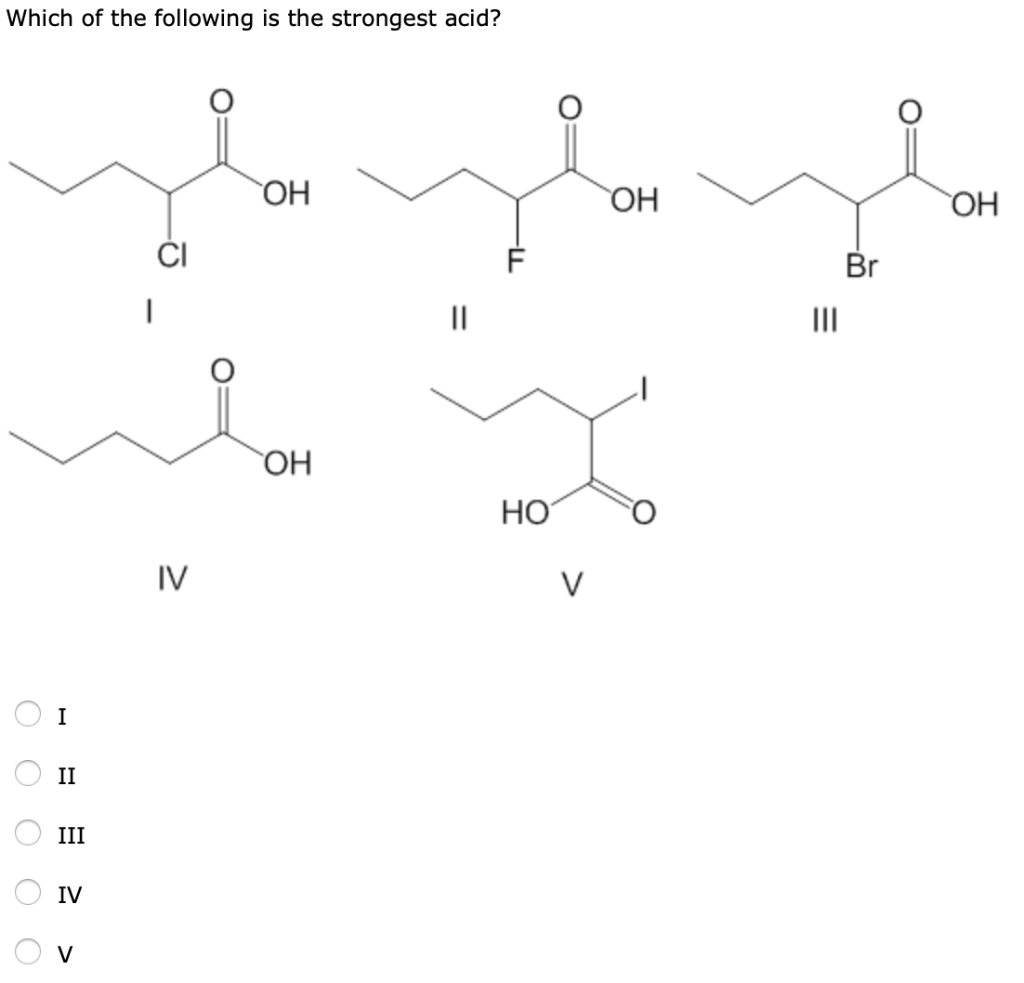 Source: chegg.com
Source: chegg.com
Fch2cooh > clch2cooh > brch2cooh > ich2cooh. 11) which of the following is the strongest acid? Which among the following is the strongest acid ?
 Source: vedantu.com
Source: vedantu.com
Based on their molecular structure, pick the stronger acid from. Fch2cooh > clch2cooh > brch2cooh > ich2cooh. H 3p o3,pka1 = 1.3,pka2 = 6.7.
 Source: transtutors.com
Source: transtutors.com
Which response includes all the acids listed below that are strong acids, and no weak acids? Nf 3 is a weaker base than : The strong acids and bases are simply those that completely dissociate in water.
Also Read :





