Hcl and ccl4 are covalent compounds; They can conduct electricity and are usually highly water soluble.
Which Of The Following Is Not An Ionic Compound. (i) kcl (ii) hcl (iii) ccl 4 (iv) nacl (a) (i) and (ii) (b) (ii) and (iii) (c) (iii) and (iv) (d) (i) and (iii) Which of the following is a typical property of an ionic compound group of answer choices? A polar covalent bond is defined as the bond which is formed when there is a difference of electronegativities between the atoms. It has a low melting point.
 Ch 11 Quiz – Bonding, Electronegativity And Lewis Structures A From studylib.net
Ch 11 Quiz – Bonding, Electronegativity And Lewis Structures A From studylib.net
Related Post Ch 11 Quiz – Bonding, Electronegativity And Lewis Structures A :
Ionic compounds have high melting point. Ionic compounds contain ions and are held together by the attractive forces among the oppositely charged ions. Which of the following is the empirical formula for the compound iron(ii) carbonate? Hence it is an ionic compound.
However, it is not always true (for example, aluminum chloride, alcl 3, is not ionic).
In chemistry, an ionic compound is a chemical compound composed of ions held together by electrostatic forces termed ionic bonding.the compound is neutral overall, but consists of positively charged ions called cations and negatively charged ions called anions.these can be simple ions such as the sodium (na +) and chloride (cl −) in sodium chloride, or polyatomic. Hence it is not an ionic compound. Here sulphur is sharing its 4 valence electrons to 4 fluorine, each fluorine is sharing its 1 valence electron with sulphur, so it’s not an ionic compound but a covalent compound. You can often recognize ionic compounds because of their properties. This guideline works well for predicting ionic compound formation for most of the compounds typically encountered in an introductory chemistry course. In chemistry, an ionic compound is a chemical compound composed of ions held together by electrostatic forces termed ionic bonding.the compound is neutral overall, but consists of positively charged ions called cations and negatively charged ions called anions.these can be simple ions such as the sodium (na +) and chloride (cl −) in sodium chloride, or polyatomic.
 Source: chem.libretexts.org
Source: chem.libretexts.org
This guideline works well for predicting ionic compound formation for most of the compounds typically encountered in an introductory chemistry course. It has a low boiling point. Ionic compounds mostly are soluble in water while the covalent compounds are not.
 Source: slideplayer.com
Source: slideplayer.com
Which of the following is a typical property of an ionic compound group of answer choices? Covalent compound is defined as the compound which is formed by the sharing of electrons between the atoms forming a compound. Hence it is an ionic compound.
 Source: doubtnut.com
Source: doubtnut.com
In chemistry, an ionic compound is a chemical compound composed of ions held together by electrostatic forces termed ionic bonding.the compound is neutral overall, but consists of positively charged ions called cations and negatively charged ions called anions.these can be simple ions such as the sodium (na +) and chloride (cl −) in sodium chloride, or polyatomic. A so 3 b c 2 h 5 oh c mgf 2 d h 2 s e nh 4 cl 5. Which of the following is true about the physical property of an ionic compound?
 Source: toppr.com
Source: toppr.com
So it is not an ionic compound. It has a low melting point. Here in carbon monoxide, carbon is sharing its 2 valence electrons & oxygen is sharing its 4 valence electrons.

Classify the following bonds as ionic, covalent, or neither o with f; It has a low boiling point. They can conduct electricity and are usually highly water soluble.
 Source: youtube.com
Source: youtube.com
Here in carbon monoxide, carbon is sharing its 2 valence electrons & oxygen is sharing its 4 valence electrons. Ionic compounds are compounds formed between a metal and nonmetal which have a crystalline lattice structure. Hardening of animal and vegetable oils
 Source: brainly.in
Source: brainly.in
It is hard but brittle. Which of the following is an example of homogeneous catalysis? In sodium chloride, sodium gives an electron to chlorine.
 Source: chegg.com
Source: chegg.com
Which of the following compounds contains both ionic and covalent bonds? Which of the following is true about the physical property of an ionic compound? The ionic compounds can form one cohesive compound, such as potassium fluoride, or form more complex polyatomic ionic compounds, such as calcium carbonate.
 Source: coursehero.com
Source: coursehero.com
Ionic bonds form only between atoms of nonmetals. Is not an ionic compound. Hence it is not an ionic compound.
 Source: chegg.com
Source: chegg.com
Which of the following is true about the physical property of an ionic compound? Because mgcl 2 is an ionic compound, and ionic compounds do not use prefixes in their names. In chemistry, an ionic compound is a chemical compound composed of ions held together by electrostatic forces termed ionic bonding.the compound is neutral overall, but consists of positively charged ions called cations and negatively charged ions called anions.these can be simple ions such as the sodium (na +) and chloride (cl −) in sodium chloride, or polyatomic.
 Source: study.com
Source: study.com
Here sulphur is sharing its 4 valence electrons to 4 fluorine, each fluorine is sharing its 1 valence electron with sulphur, so it’s not an ionic compound but a covalent compound. Metallic compounds contain freely floating electrons which allow them to conduct electricity and heat well. What is the charge on the metal, m?

What is the charge on the metal, m? Which of the following is not an ionic compound? A so 3 b c 2 h 5 oh c mgf 2 d h 2 s e nh 4 cl 5.
 Source: toppr.com
Source: toppr.com
Many covalent compounds are flexible or gaseous and are not water soluble. In chemistry, an ionic compound is a chemical compound composed of ions held together by electrostatic forces termed ionic bonding.the compound is neutral overall, but consists of positively charged ions called cations and negatively charged ions called anions.these can be simple ions such as the sodium (na +) and chloride (cl −) in sodium chloride, or polyatomic. Only statement 1 is true.
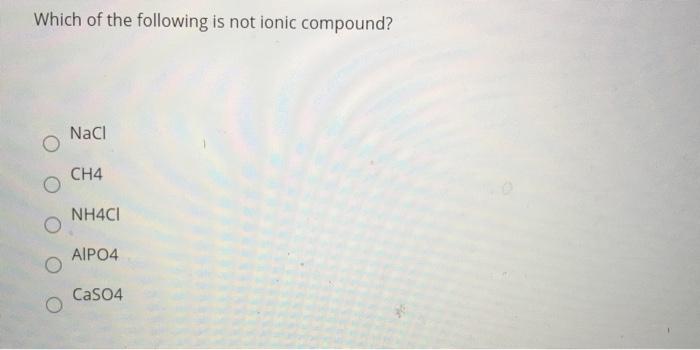
Ionic compounds contain ions and are held together by the attractive forces among the oppositely charged ions. A) lif b) ccl 4 c) nh 4 cl d) feso 4. Only statement 2 is true.
 Source: numerade.com
Source: numerade.com
In sodium chloride, sodium gives an electron to chlorine. The ionic compounds can form one cohesive compound, such as potassium fluoride, or form more complex polyatomic ionic compounds, such as calcium carbonate. Example:, a covalent bond is formed by sharing of electrons between non metals.
 Source: clutchprep.com
Source: clutchprep.com
They can conduct electricity and are usually highly water soluble. Hence it is not an ionic compound. Which of the following is a typical property of an ionic compound group of answer choices?
 Source: youtube.com
Source: youtube.com
It has a low melting point. They can conduct electricity and are usually highly water soluble. Example:, a covalent bond is formed by sharing of electrons between non metals.
 Source: youtube.com
Source: youtube.com
Two pure elements react to form a compound. Two pure elements react to form a compound. It is soft and brittle.
 Source: chegg.com
Source: chegg.com
Ionic bond is formed when there is complete transfer of electron from metal to nonmetal. Hence we can conclude, calcium carbide can be classified as a compound with ionic nature. Hardening of animal and vegetable oils
 Source: studylib.net
Source: studylib.net
Furthermore, what is the name of the following compound mgcl2 5h2o?, magnesium chloride hexahydrate (mgcl2. So, all discussions are shown above, it means carborundum is. The ionic compounds can form one cohesive compound, such as potassium fluoride, or form more complex polyatomic ionic compounds, such as calcium carbonate.
Also Read :