(i) ch 3 ch 2 ch 3. L i +, n a +, k +, c s +, r b + are soluble.
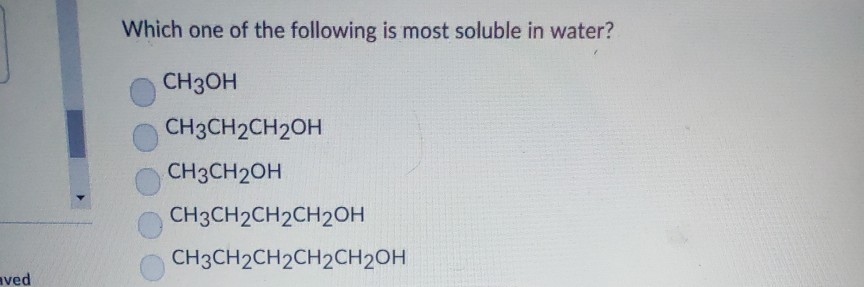
Which Of The Following Is Most Soluble In Water. Ch 3 ch 2 nh 2. Some of the exceptions are c a s o 4, b a s o 4, p b s o 4, a g 2 s o 4 and s r s o 4. The metallurgical process in which a met. Among given compounds, ethylene glycol ( ho−ch2−ch2−oh ) is the most soluble in water.
Related Post Solved Which One Of The Following Is Most Soluble In Water? | Chegg.com :
Calculate simultaneous solubility of agcn s and agbr in a solution of water. Building line following and food following robotsbuilding line following and food following ; Rank from most to least soluble in water. Ethylene glycol has two hydroxy groups both of which form hydrogen bonds with water.
Which of the following orbitals has maxi.
Which aldehyde is most soluble in water? [ksp of agbr=5×10−13 and ksp of agcn s=1×10−12 ]. All sodium, potassium and ammonium salts are soluble in water. Greater is the number of hydrogen bonds, greater is the extent of hydrogen bonding and greater is the solubility in water. For example, hexanol (ch3ch2ch2ch2ch2ch2oh), is only very slightly soluble in water (0.4 g/l). Salts containing nitrate ion i.e.
 Source: youtube.com
Source: youtube.com
Greater is the number of hydrogen bonds, greater is the extent of hydrogen bonding and greater is the solubility in water. Is methanol the most soluble in water? Rank from most to least soluble in water.
 Source: toppr.com
Source: toppr.com
The main buffer system of the human bloo. Ethylene glycol has two hydroxy groups both of which form hydrogen bonds with water. Formaldehyde, acetaldehyde, and acetone are soluble in water.
 Source: youtube.com
Source: youtube.com
The most commonly used bleaching agent. Pbcl2, pbbr2, and pbi2 are soluble in hot water. Further there are few exceptions to this rule.
![Solved] Which Of The Following Compounds Would Be The Most Soluble In Water? | Course Hero](https://www.coursehero.com/qa/attachment/15926613/ “Solved] Which Of The Following Compounds Would Be The Most Soluble In Water? | Course Hero”) Source: coursehero.com
Which among the following is popularly c. Ksp of agcn s=1×10−12, ksp of agbr=5×10−13]. Ch 3 ch 2 ch 2 ch 2 nh 2.
 Source: clutchprep.com
Source: clutchprep.com
The relation between solubility and solubility product constant is quite important when describing the solubility of slightly ionic compounds. Among c s c l o 4, n c l o 4 and l i c l o 4 all of the cations have + 1 charge, so for comparing hydration energy size factor will be considered smaller is the size more will be the hydration energy. (ii) ch 3 ch 2 ch 2 ch 3.

(v) ch 3 ch 2 ch 2 ch 2 ch 2 ch 2 ch 2 ch 3. 5 important lessons you should learn during this worldwide…. Ksp of agcn s=1×10−12, ksp of agbr=5×10−13].
 Source: doubtnut.com
Source: doubtnut.com
Which of the following is the most soluble in water? A solution that contains 50 g of nh₄cl in 100 g of water at 50°c is _____ (saturated, unsaturated, supersaturated). 5 important lessons you should learn during this worldwide….
 Source: chegg.com
Source: chegg.com
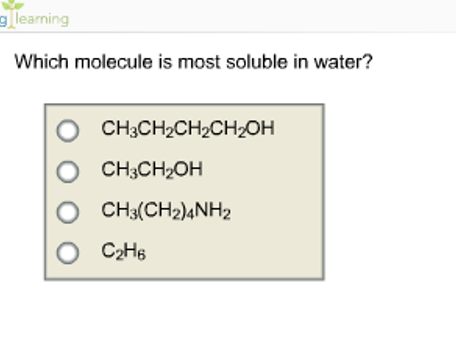
Alcohols contain an oh group in which a hydrogen atom is attached to the electronegative oxygen atom, due to which it is capable of forming hydrogen bonds with the water molecule leading to greater solubility of alcohol in water. Thus, among all cations l i + is smallest in size. Ch 3 ch 2 nh 2.
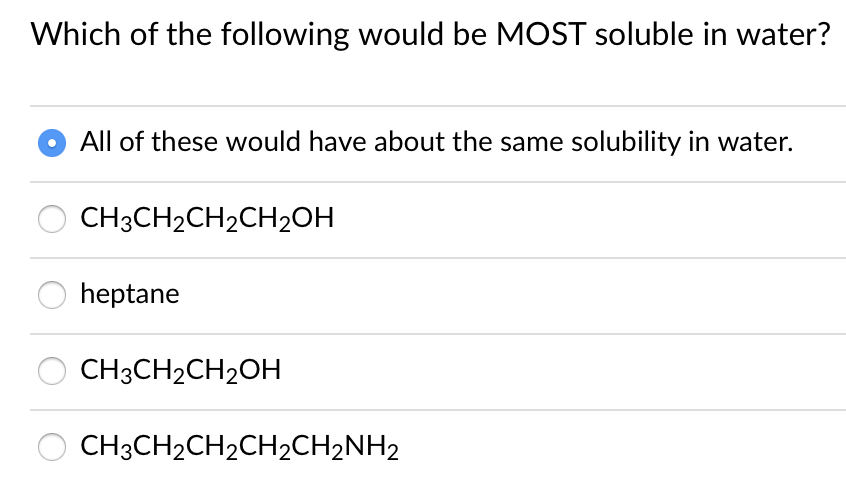 Source: bartleby.com
Source: bartleby.com
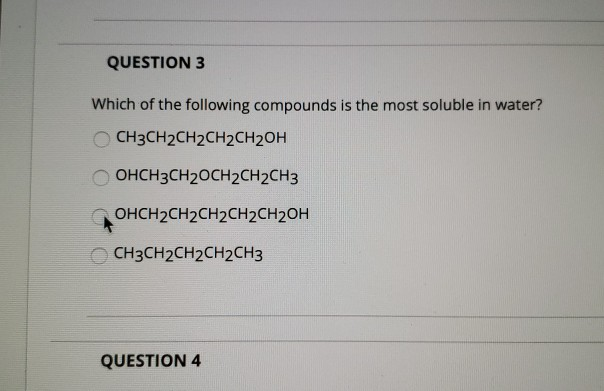
Which of the following is the most soluble in water? Which of the following phenomenon is con. Sharing of 1 electron pair by one specie.

(ii) ch 3 ch 2 ch 2 ch 3. Which of the following molecules is the most soluble in water? Building line following and food following robotsbuilding line following and food following ;
 Source: chegg.com
Source: chegg.com
Rank the following substances in order from most soluble in water to least soluble in water: Which among the following is popularly c. Which of the following orbitals has maxi.
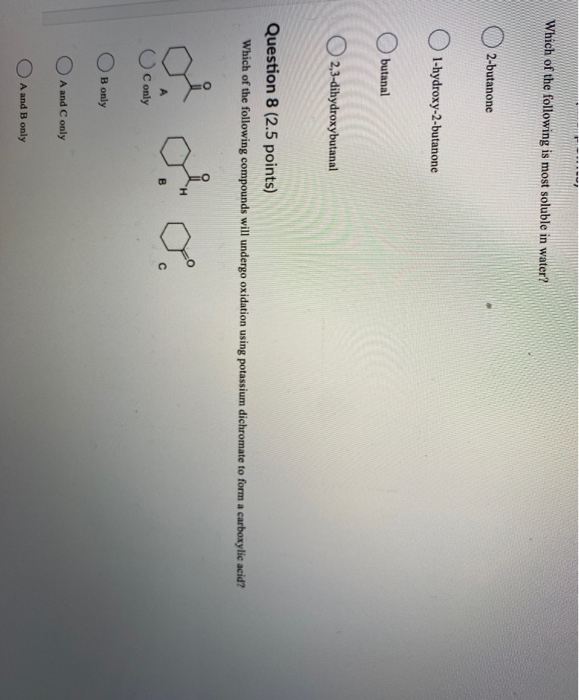 Source: chegg.com
Source: chegg.com
( n o 3 −) are generally soluble. Formaldehyde, acetaldehyde, and acetone are soluble in water. (v) ch 3 ch 2 ch 2 ch 2 ch 2 ch 2 ch 2 ch 3.
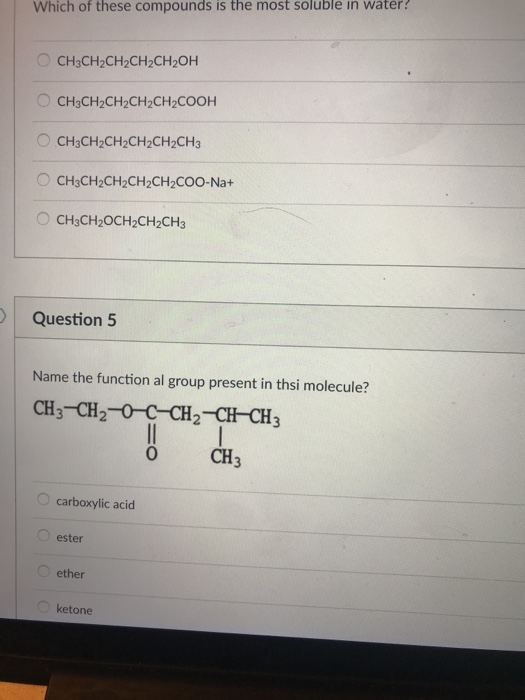 Source: chegg.com
Source: chegg.com
Hgi2 is insoluble in water. Which of the following is produced when. Calculate simultaneous solubility of agcns and agbr in a solution of water.
 Source: toppr.com
Source: toppr.com
Building line following and food following robotsbuilding line following and food following ; [ksp of agbr=5×10−13 and ksp of agcn s=1×10−12 ]. Hgi2 is insoluble in water.
 Source: toppr.com
Source: toppr.com
Which of the following compounds would you expect to be most soluble in water? Rank from most to least soluble in water. (ii) ch 3 ch 2 ch 2 ch 3.
 Source: oneclass.com
Source: oneclass.com
Ch 3 ch 2 nh 2. Which of the following compound is most soluble in water? Hence, zns is the most soluble.
 Source: youtube.com
Source: youtube.com
Thus, among all cations l i + is smallest in size. A complete resource book in chemistry. (iv) ch 3 ch 2 ch 2 oh.
 Source: toppr.com
Source: toppr.com
(iv) ch 3 ch 2 ch 2 oh. Rank the following substances in order from most soluble in water to least soluble in water: Calculate the solubility ‘s’ for each option, higher the value of ‘s’ higher the solubility.
 Source: youtube.com
Source: youtube.com
A solution that contains 50 g of nh₄cl in 100 g of water at 50°c is _____ (saturated, unsaturated, supersaturated). Calculate simultaneous solubility of agcns and agbr in a solution of water. (iii) ch 3 ch 2 ch 2 ch 2 oh.
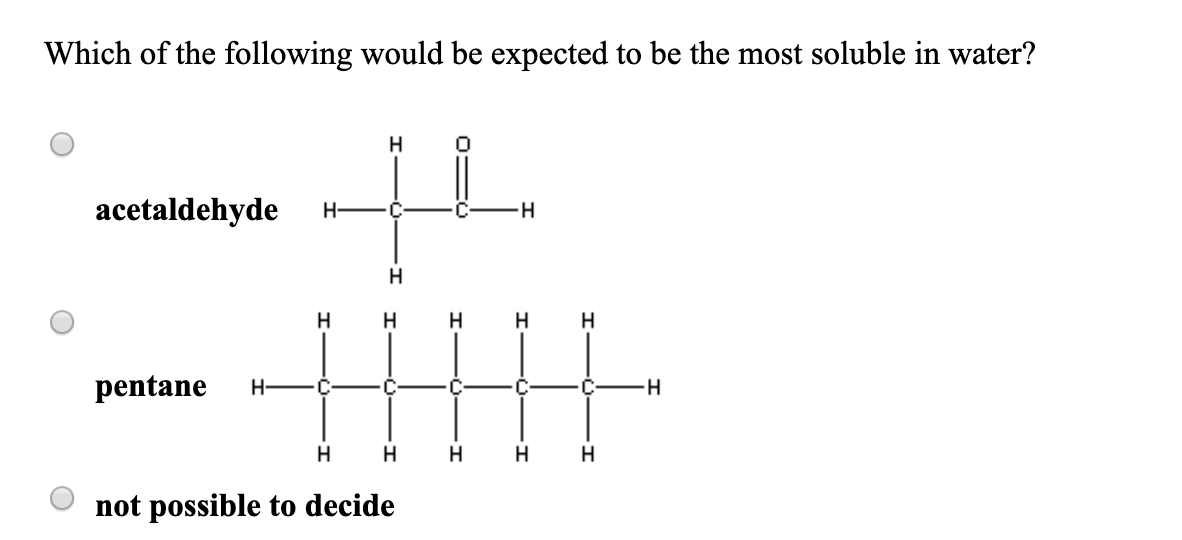 Source: chegg.com
Source: chegg.com
Greater is the number of hydrogen bonds, greater is the extent of hydrogen bonding and greater is the solubility in water. Greater is the number of hydrogen bonds, greater is the extent of hydrogen bonding and greater is the solubility in water. Which of the following is produced when.
Also Read :





