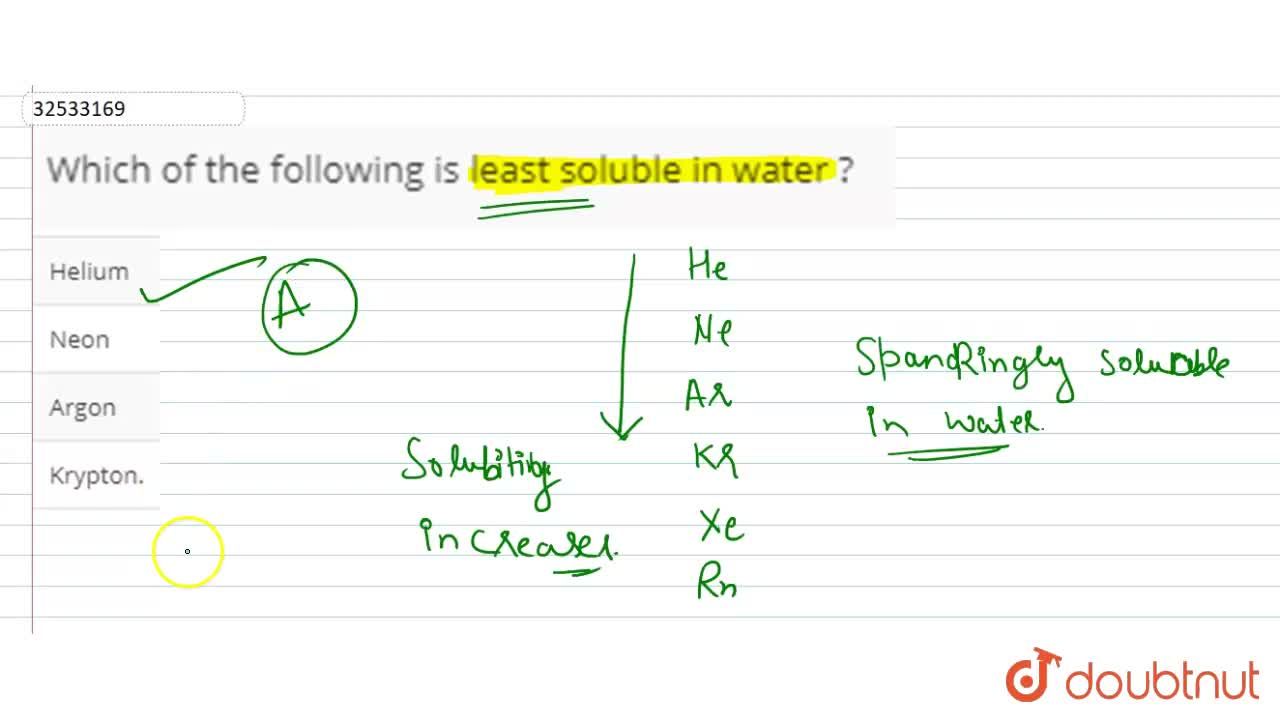
Which of the following is least soluble in water ? Among the following compounds, the strontium fluoride has the least solubility.
Which Of The Following Is Least Soluble In Water. So for this, we need to draw the lewis structures of the given choices and determine their polarity. As we can see the solubility nature of chemicals we can say that bef2 and mgcl2 are is least soluble than as the other two i.e, caf2, mgf2 are insoluble in water like comment Which salt is least soluble in water. Which butyl alcohol is completely soluble in water?
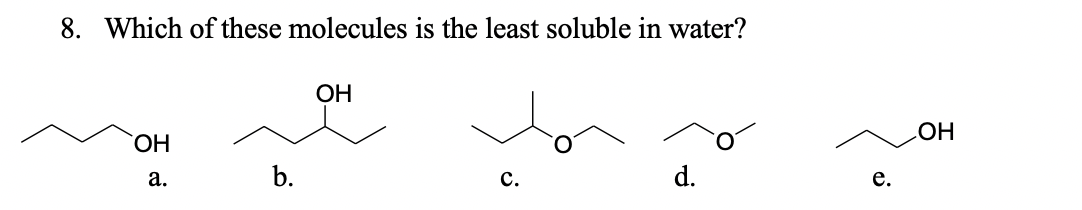 Answered: 8. Which Of These Molecules Is The… | Bartleby From bartleby.com
Answered: 8. Which Of These Molecules Is The… | Bartleby From bartleby.com
Related Post Answered: 8. Which Of These Molecules Is The… | Bartleby :
All are soluble in water except mgs, magnesium sulphide. Which compound would be least soluble in water? Thus, the least soluble compound is hgs. View available hints) reset help magnesium chlonde 1.
A) iron (iii) chloride b) ammonium acetate c) sodium hydroxide d) magnesium carbonate.
2 show answers another question on chemistry. Thus, the least soluble compound is hgs. Which of the following compounds is the least soluble in water? 2 show answers another question on chemistry. Mgs (s) + 2h2o (l) = mg(oh)2 (s) + h2s (g) sodium salts are generally soluble in water. Rank from most to least soluble in water.
![Solved] Which Would Be The Least Soluble In Water? | Course Hero](https://www.coursehero.com/qa/attachment/16285556/ “Solved] Which Would Be The Least Soluble In Water? | Course Hero”) Source: coursehero.com
2 show answers another question on chemistry. How many grams of potassium chloride can be dissolved in 200 g of water at 80° c? Among the following compounds, the strontium fluoride has the least solubility.
 Source: youtube.com
Source: youtube.com
Rank the following substances in order from most soluble in water to least soluble in water: Rank from most to least soluble in water. Rank the following substances in order from most soluble in water to least soluble in water:
 Source: youtube.com
Source: youtube.com
On the right here we question: All are soluble in water except mgs, magnesium sulphide. Due to lack of hydrogen bonding, alkane is least soluble inter.
 Source: embibe.com
Source: embibe.com
Which one of the following is least soluble in water? Sodium chloride is the least soluble in which of the following liquids? H1 which of the following will be most soluble in water at 2 q c?
 Source: neetlab.com
Source: neetlab.com
(a) (ch3)2nh (b) c6h5nh2 (c) ch3nh2 (d) (ch3)3n. Ozone is used for purifying water because. Due to lack of hydrogen bonding, alkane is least soluble inter.
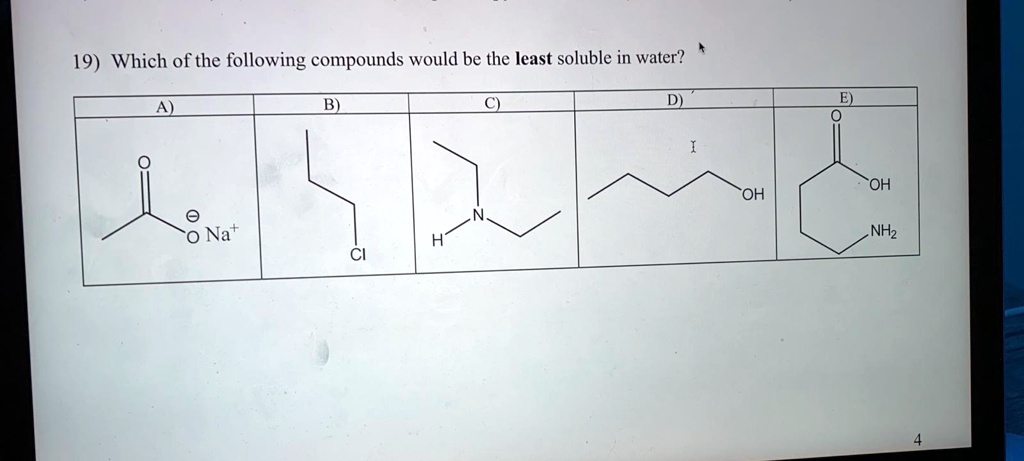 Source: numerade.com
Source: numerade.com
Which of the following is least soluble in water. Which butyl alcohol is completely soluble in water? A) ch3oh b) ch3ch2ch2oh c) ch3ch2oh d) ch3ch2ch2ch2oh e) ch3ch2ch2ch2ch2oh question 2 the standard emf for the cell using the overall cell reaction below is +0.48 v:
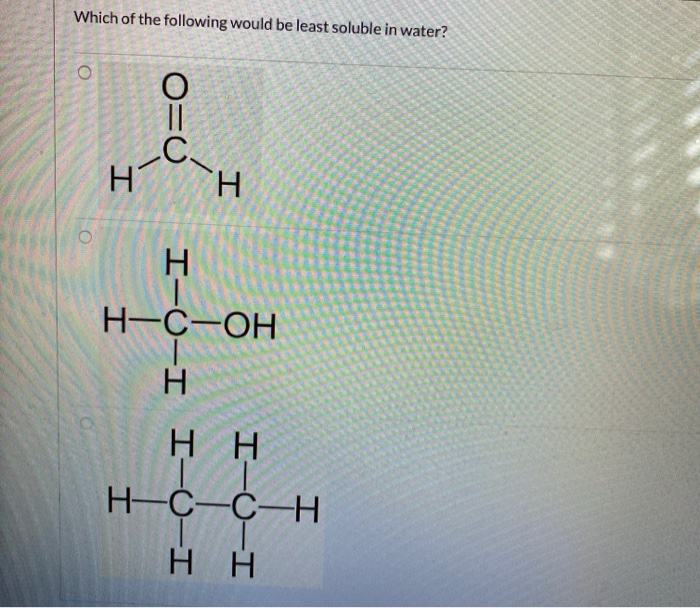 Source: chegg.com
Source: chegg.com
My guess is b because it�s nonpolar and i think it�s a london dispersion imf. If the supply of oxygen is limited, h 2 s reacts with o 2 to form. Rank the following organic compounds from most soluble in water to least soluble in water.
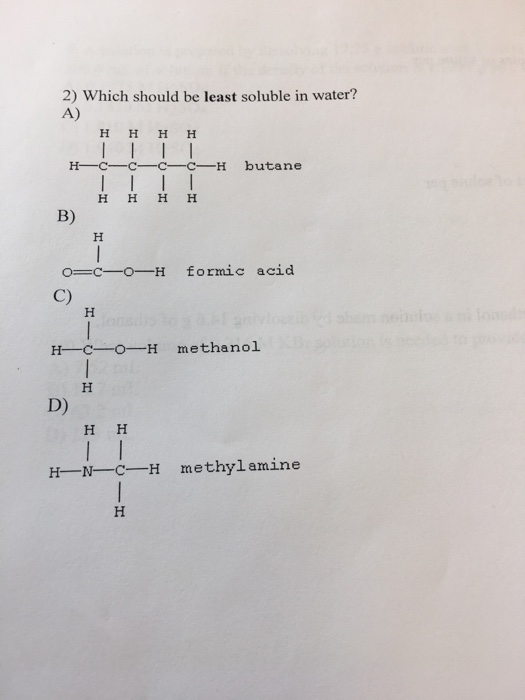
Rank the following substances in order from most soluble in water to least soluble in water: Which one of the following is least soluble in water? Barium sulphate is insoluble in water because it has a high lattice energy.
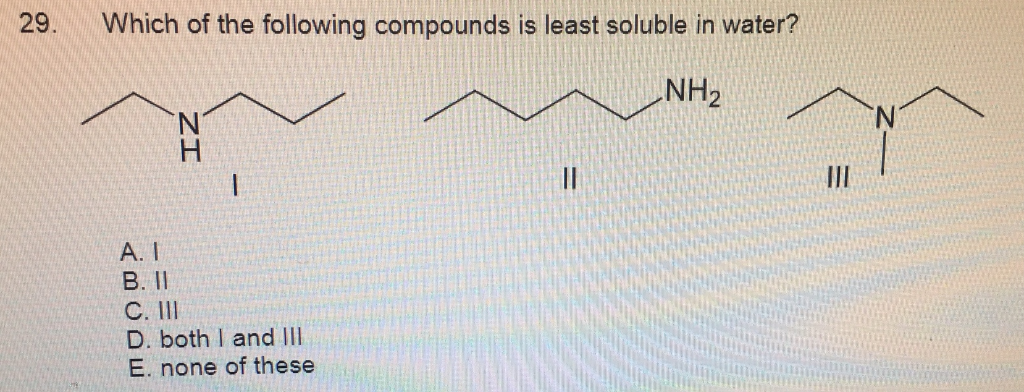 Source: chegg.com
Source: chegg.com
Which compound would be least soluble in water? Rank the following substances in order from most soluble in water to least soluble in water: So,they are must soluble in water.nh3 is a polar covalent compound which is soluble in polar solvent water.
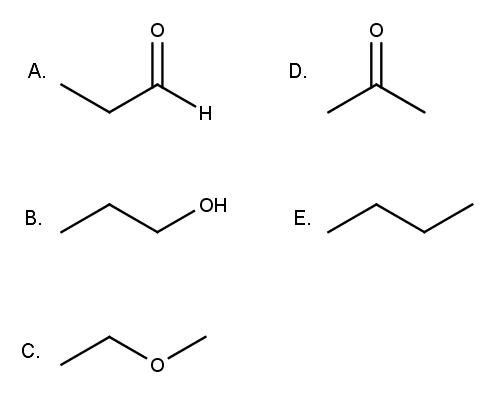 Source: clutchprep.com
Source: clutchprep.com
Zn (s) + ni2+ (aq) zn2+ (aq) + ni (s) the emf generated by the cell when ni2+ = 0.100 m and zn2+ = 2.25 m is a) 0.56 b) 0.50 c) 0.44 Rank the following organic compounds from most soluble in water to least soluble in water. Which of the following compounds is least soluble in water?
 Source: brainly.com
Source: brainly.com
So,they are must soluble in water.nh3 is a polar covalent compound which is soluble in polar solvent water. Rank from most to least soluble in water. • view available hint (s) reset help propanol methane.

Which is least soluble in water among the following? View available hints) reset help magnesium chlonde 1. And ethane, c 2 h 6.
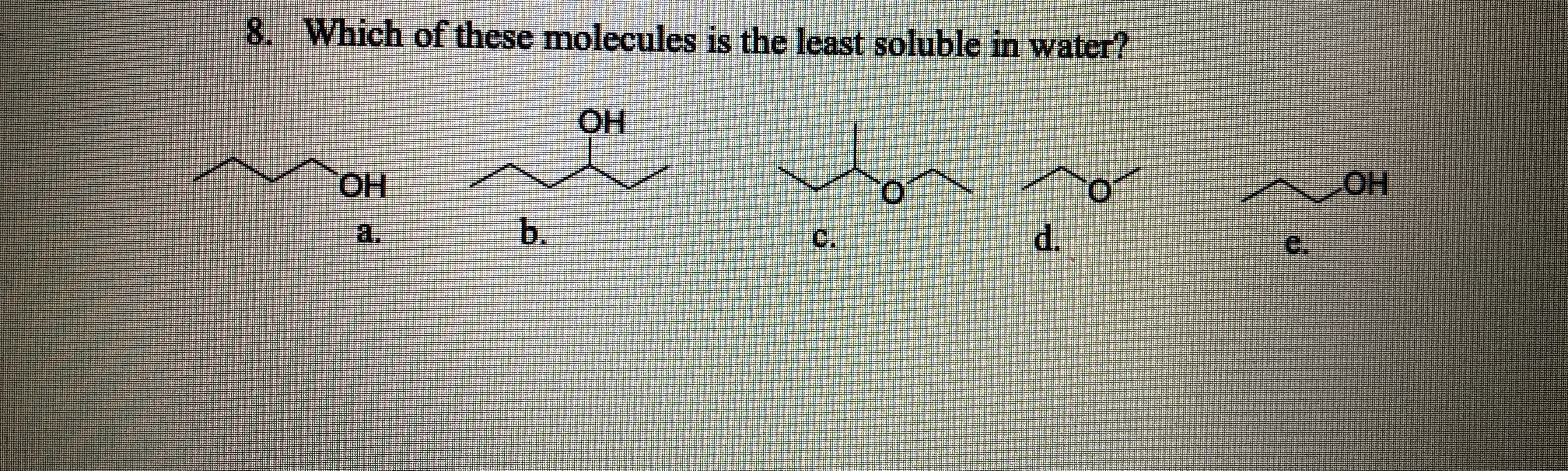 Source: bartleby.com
Source: bartleby.com
Except lithium hydroxide,all the alkali metal hydroxides are highly soluble in water.lioh is much less soluble on account of high lattice enthalpy. So, agi is least soluble of the given options. 1) acetone 2) formaldehyde 3) acetaldehyde 4) benzaldehyde please explain the answer also thank you
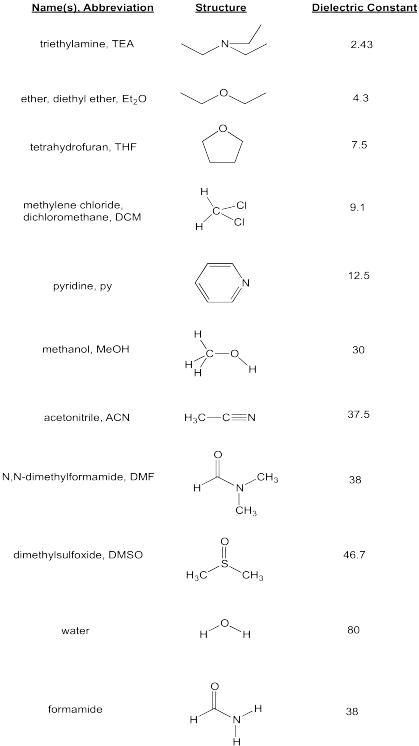 Source: employees.csbsju.edu
Source: employees.csbsju.edu
H1 which of the following will be most soluble in water at 2 q c? Which of the following compounds is least soluble in water? Determine the central atom in this molecule.
 Source: clutchprep.com
Source: clutchprep.com
A)nacl b)kcl c)nh4cl d)hcl 7.according to reference table g, which of the following substances is least soluble in 100 grams of water at 50ºc? Which of the following is least soluble in water ? Zn (s) + ni2+ (aq) zn2+ (aq) + ni (s) the emf generated by the cell when ni2+ = 0.100 m and zn2+ = 2.25 m is a) 0.56 b) 0.50 c) 0.44
 Source: bartleby.com
Source: bartleby.com
Which salt is least soluble in water. On adding water in barium sulphate its hydration energy decreases more rapidly in comparison to its lattice energy. • view available hint (s) reset help propanol methane.
 Source: youtube.com
Source: youtube.com
To rank items as equivalent, overlap them. Mgs is easily decomposed (hydrolysed) by water to form insoluble magnesium hydroxide and hydrogen sulphide gas. Among the following compounds, the strontium fluoride has the least solubility.
 Source: toppr.com
Source: toppr.com
Due to lack of hydrogen bonding, alkane is least soluble inter. Mgs (s) + 2h2o (l) = mg(oh)2 (s) + h2s (g) sodium salts are generally soluble in water. Which compound would be least soluble in water?
 Source: doubtnut.com
Source: doubtnut.com
• view available hint (s) reset help propanol methane. Rank the following organic compounds from most soluble in water to least soluble in water. Therefore, for any compound to become soluble in water, it should be polar or have hydrogen bonding in it.

Why baso4 is not soluble in water? A)kno3 b)ki c)nh3 d)nacl 8.according to table g, which substance forms an Alcohols, carboxylic acids, carboxylic acid chlorides, amines, esters are usually soluble in water.
Also Read :





