I) all sulfides are insoluble except those of ammonium, sodium, calcium, potassium, magnesium, barium and strontium. D) the primary nitrogenous waste product of most birds.
Which Of The Following Is Insoluble In Water. This cookie is set by gdpr cookie consent plugin. All ionic compounds are soluble in water to some extent, but the degree of solubility varies. Predict whether the following compounds are soluble or insoluble in water:nh4br, cubr2, agl, pb(no3)2, cuco3, caso4 Helium is an unreactive gas and neon is a gas of extremely low reactivity.
 Solubility And Precipitation | Bioprofe From bioprofe.com
Solubility And Precipitation | Bioprofe From bioprofe.com
Related Post Solubility And Precipitation | Bioprofe :
14.the dissolving of solid lithium bromide in water is represented by the balanced equation below. E) the primary nitrogenous waste. D) the primary nitrogenous waste product of most birds. Classify each of the following as a strong electrolyte or non electrolyte.mgbr2, c12h22o11, na2co3, koh q.
Potassium sulfate, or k_2so_4, si soluble because all sulfates are soluble with the exception of those formed with silver, calcium, barium, mercury, lead, and strontium ions, finally, copper (ii) hydroxyde, or cu(oh)_2, is insoluble because all hydroxides are insoluble with the exception of those formed with alkali metal ions and barium.
This is an exception as halide salt of most of the metal are soluble in water. 9) which of the following is true of urea? Which of the following is a key difference between sweepstakes and contests? All of the above which of the following. 14.the dissolving of solid lithium bromide in water is represented by the balanced equation below. Predict whether the following compounds are soluble or insoluble in water:nh4br, cubr2, agl, pb(no3)2, cuco3, caso4
 Source: science-sparks.com
Source: science-sparks.com
It is a) insoluble in water. Get why is ethyl 4 aminobenzoate insoluble in water from 6 different pptx ethyl 4 aminobenzoate was found to be insoluble in water and 10 m naoh but upon addition of 60 m naoh to this solution ethyl 4 aminobenzoate became insoluble. Which of the following is a key difference between sweepstakes and contests?

Which of the following is a key difference between sweepstakes and contests? D) the primary nitrogenous waste product of most birds. Calcium carbonate is insoluble in water because the electrostatic bonds between the carbonate anion and the calcium ion are too strong to be overcome by solvation by water molecules.

5when ethyl 4 aminobenzoate was added into 1. Which one of the following compounds is insoluble in water? Predict whether the following compounds are soluble or insoluble in water:nh4br, cubr2, agl, pb(no3)2, cuco3, caso4

For example, sugar, common salt, glucose and vinegar are soluble in water, while kerosene oil, petrol and edible oils are insoluble in water and therefore form a separate layer on water. Get why is ethyl 4 aminobenzoate insoluble in water from 6 different pptx ethyl 4 aminobenzoate was found to be insoluble in water and 10 m naoh but upon addition of 60 m naoh to this solution ethyl 4 aminobenzoate became insoluble. A.cal 2 b.na so 2 4 c.agf d.ai(oh) 3 2 see answers advertisement advertisement histrionicus histrionicus answer:
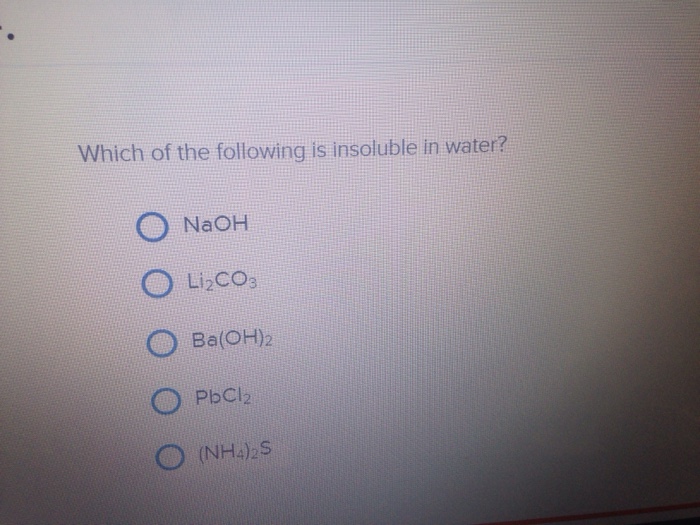
Check answer and solution f Therefore, and are insoluble in water. Which of the following is a key difference between sweepstakes and contests?
 Source: bioprofe.com
Source: bioprofe.com
Which of the following compounds is insoluble in water? Predict whether the following compounds are soluble or insoluble in water:nh4br, cubr2, agl, pb(no3)2, cuco3, caso4 B) more toxic to human cells than ammonia.
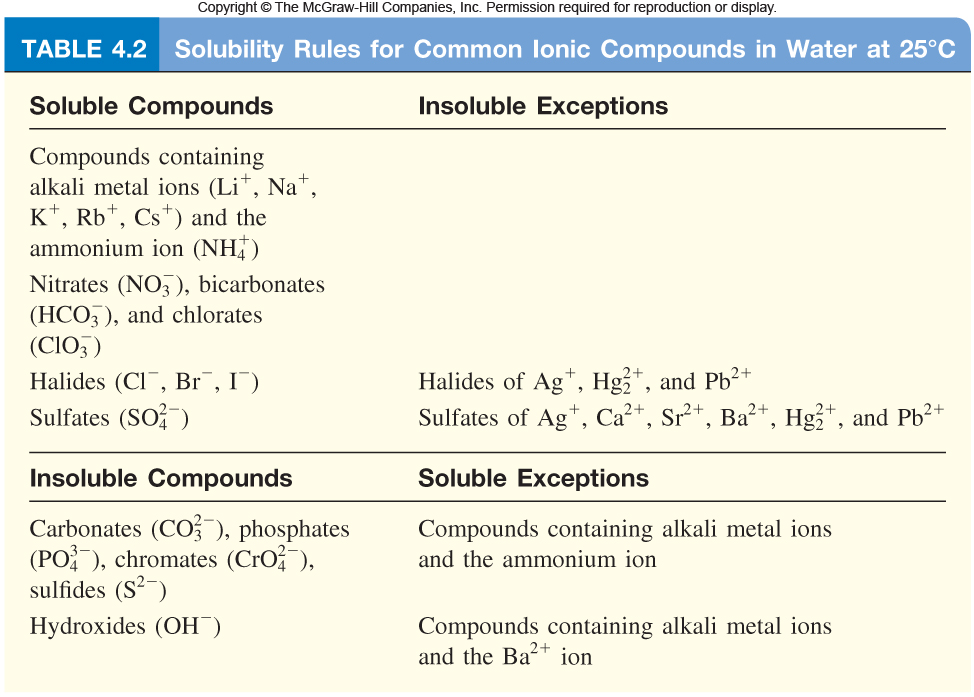 Source: socratic.org
Source: socratic.org
All of these are slightly hydrolyzed in water. Sodium is an electrolyte that regulates the amount of water in your body. Predict whether the following compounds are soluble or insoluble in water:nh4br, cubr2, agl, pb(no3)2, cuco3, caso4

This cookie is set by gdpr cookie consent plugin. Haemoglobin, ribonuclease and adenine are either soluble in cold or hot water. Sodium sulfide ammonium sulfate barium nitrate potassium phosphate
 Source: ask.learncbse.in
Source: ask.learncbse.in
Which one of the following compounds is insoluble in water? B) more toxic to human cells than ammonia. A.cal 2 b.na so 2 4 c.agf d.ai(oh) 3 2 see answers advertisement advertisement histrionicus histrionicus answer:
 Source: chegg.com
Source: chegg.com
Get why is ethyl 4 aminobenzoate insoluble in water from 6 different pptx ethyl 4 aminobenzoate was found to be insoluble in water and 10 m naoh but upon addition of 60 m naoh to this solution ethyl 4 aminobenzoate became insoluble. Predict whether the following compounds are soluble or insoluble in water:nh4br, cubr2, agl, pb(no3)2, cuco3, caso4 D) the primary nitrogenous waste product of most birds.
 Source: chegg.com
Source: chegg.com
Potassium sulfate, or k_2so_4, si soluble because all sulfates are soluble with the exception of those formed with silver, calcium, barium, mercury, lead, and strontium ions, finally, copper (ii) hydroxyde, or cu(oh)_2, is insoluble because all hydroxides are insoluble with the exception of those formed with alkali metal ions and barium. This cookie is set by gdpr cookie consent plugin. 9) which of the following is true of urea?
 Source: chegg.com
Source: chegg.com
The halide salts of silver and lead are also insoluble in water. ((po4)2 is usually insoluble unless paired with li, na, k, or nh4 in water) identify the solid product formed, if any, from the reaction of (nh4)2co3 and mg(no3)2. Haemoglobin, ribonuclease and adenine are either soluble in cold or hot water.
 Source: toppr.com
Source: toppr.com
A.cal 2 b.na so 2 4 c.agf d.ai(oh) 3 2 see answers advertisement advertisement histrionicus histrionicus answer: All of the above which of the following. Calculate the volume of koh required to reach the equivalence point.

All of these are slightly hydrolyzed in water. The cookie is used to store the user consent for the cookies in the category analytics. A volume of 40.0 ml of a 0.410 m hno3 solution is titrated with 0.110 m koh.
 Source: kolekcijablogs.blogspot.com
Source: kolekcijablogs.blogspot.com
It is a) insoluble in water. Practice exercise 1 which of the following compounds is insoluble in water? Which of the following compounds is insoluble in water?
 Source: chegg.com
Source: chegg.com
In the modem periodic table, which are the metals among the first ten elements. Therefore, and are insoluble in water. This cookie is set by gdpr cookie consent plugin.
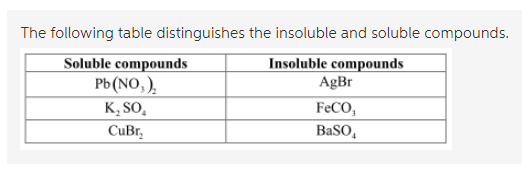 Source: ask.learncbse.in
Source: ask.learncbse.in
B) more toxic to human cells than ammonia. 9) which of the following is true of urea? Get why is ethyl 4 aminobenzoate insoluble in water from 6 different pptx ethyl 4 aminobenzoate was found to be insoluble in water and 10 m naoh but upon addition of 60 m naoh to this solution ethyl 4 aminobenzoate became insoluble.

(a) (nh4)2s, (b) caco3, (c) naoh, (d) ag2so4 (e) pb(ch3coo)2 (a) (nh4)2s, (b) caco3, (c) naoh, (d) ag2so4 (e) pb(ch3coo)2 Sodium is an electrolyte that regulates the amount of water in your body. (a) (nh4)2s, (b) caco3, (c) naoh, (d) ag2so4 (e) pb(ch3coo)2 (a) (nh4)2s, (b) caco3, (c) naoh, (d) ag2so4 (e) pb(ch3coo)2
 Source: slideplayer.com
Source: slideplayer.com
B) more toxic to human cells than ammonia. All of the above which of the following. Solved 1 which one of the following is characteristic of chegg.
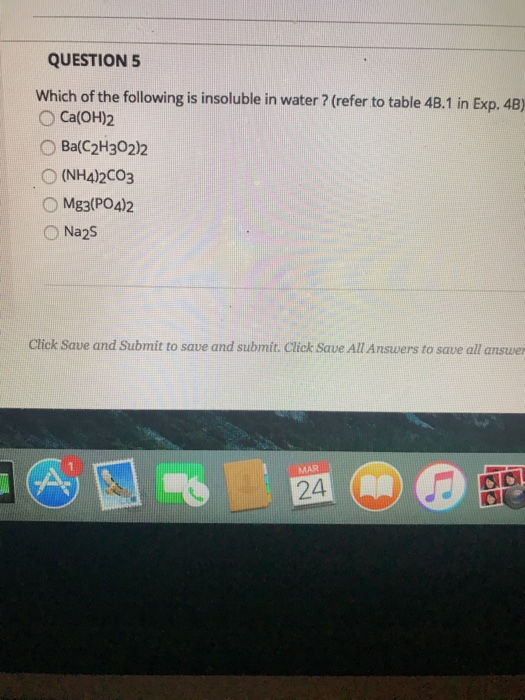 Source: chegg.com
Source: chegg.com
Potassium sulfate, or k_2so_4, si soluble because all sulfates are soluble with the exception of those formed with silver, calcium, barium, mercury, lead, and strontium ions, finally, copper (ii) hydroxyde, or cu(oh)_2, is insoluble because all hydroxides are insoluble with the exception of those formed with alkali metal ions and barium. Check answer and solution f 14.the dissolving of solid lithium bromide in water is represented by the balanced equation below.
Also Read :