All of the above are true. Which one of the following reactions is not an exothermic (a) absorption of sulphur troixide by 98.5% sulphuric acid (b) oxidation of sulphur trioxide (c) oxidation of sulphur to sulphur dioxide (d) thermal dissociation of iron pyrites
Which Of The Following Is An Exothermic Reaction. So, this reaction is an exothermic reaction. Matching a light using a matchstick is one example of this type of reaction where. A few examples are neutralization, burning a substance, reactions of fuels, deposition of dry ice, respiration, solution of sulfuric acid into water and much more. Expressed in a chemical equation:
 Which Of The Following Describes An Exothermic Reaction? - Brainly.com From brainly.com
Which Of The Following Describes An Exothermic Reaction? - Brainly.com From brainly.com
Related Post Which Of The Following Describes An Exothermic Reaction? - Brainly.com :
The substance that loses the electrons is said to be oxidized, while the substance that gains the electrons is said to be reduced. In an exothermic reaction, heat is released. Melting ice is an endothermic process. An exothermic reaction is a reaction in which energy is released in the form of light or heat.
Generally, decomposition reactions are endothermic reactions.
Since every chemical change involves a gain or loss of energy. Thus in an exothermic reaction, energy is transferred into the surroundings rather than taking energy from the surroundings as in an endothermic reaction. When energy is transferred as heat from the surroundings to the system, δh is negative.e. A photosynthesis is an endothermic reaction, so more energy is absorbed making bonds than is released breaking bonds. Example of a temperature change that might occur in an endothermic reaction. It is neither endothermic nor exothermic.
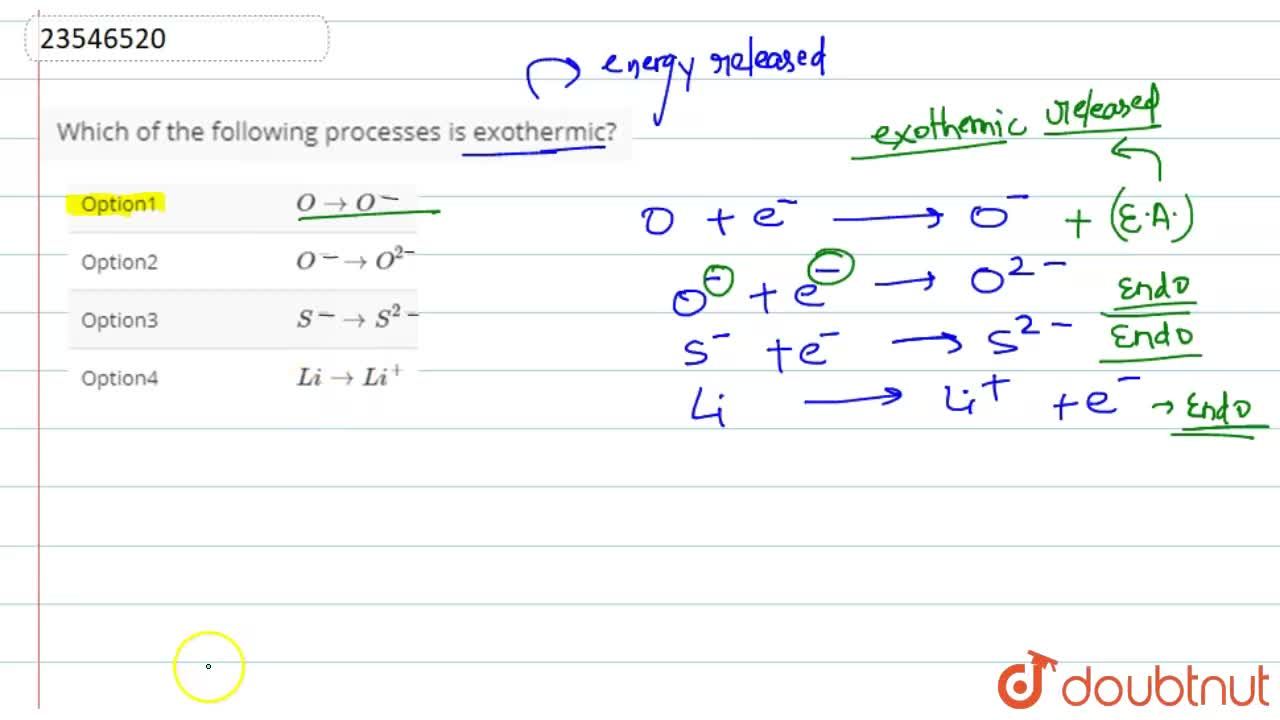 Source: doubtnut.com
Source: doubtnut.com
The reaction in which heat is absorbed is called an endothermic reaction. It is neither endothermic nor exothermic. Heat is needed to be supplied for the decomposition of calcium carbonate to form quicklime and co 2 , so it is an endothermic reaction.
 Source: youtube.com
Source: youtube.com
A reaction which absorbs energy is endothermic. What is an exothermic reaction? Which of the following statements is true?
 Source: teachoo.com
Source: teachoo.com
A few examples are neutralization, burning a substance, reactions of fuels, deposition of dry ice, respiration, solution of sulfuric acid into water and much more. A photosynthesis is an endothermic reaction, so more energy is absorbed making bonds than is released breaking bonds. It is the opposite of an endothermic reaction.
 Source: slideserve.com
Source: slideserve.com
Exothermic reaction means exo meaning releases and thermic means heat. So, this reaction is an exothermic reaction. Therefore, respiration and burning of a candle are examples of exothermic reaction whereas evaporation of water and melting of ice are examples of endothermic reaction.

Which of the following statements is true? In an endothermic reaction, heat is absorbed. What is an exothermic reaction?
 Source: toppr.com
Source: toppr.com
The following reactions are exothermic in an exothermic reaction, heat has been released to the surroundings from the system. Heat is needed to be supplied for the decomposition of calcium carbonate to form quicklime and co 2 , so it is an endothermic reaction. Respiration is the breakdown of food to release energy.
 Source: clutchprep.com
Source: clutchprep.com
These are reactions that transfer energy to the surroundings (ie the energy ex its from the reaction, hence the name ex othermic). Therefore, respiration and burning of a candle are examples of exothermic reaction whereas evaporation of water and melting of ice are examples of endothermic reaction. 1) oxidation of iron involves reaction of elemental iron with oxygen to form iron oxide (rust).
 Source: toppr.com
Source: toppr.com
The evaporation of water is an exothermic process.f. Quick lime reacts vigorously with water releasing a. This reaction releases heat, hence, an exothermic reaction.
 Source: chegg.com
Source: chegg.com
When energy is transferred as heat from the surroundings to the system, delta h is negative. This reaction releases heat, hence, an exothermic reaction. Example of a temperature change that might occur in an endothermic reaction.
 Source: brainly.com
Source: brainly.com
- oxidation of iron involves reaction of elemental iron with oxygen to form iron oxide (rust). Therefore, it can be understood that the net amount. During this reaction, a large amount of heat is released.
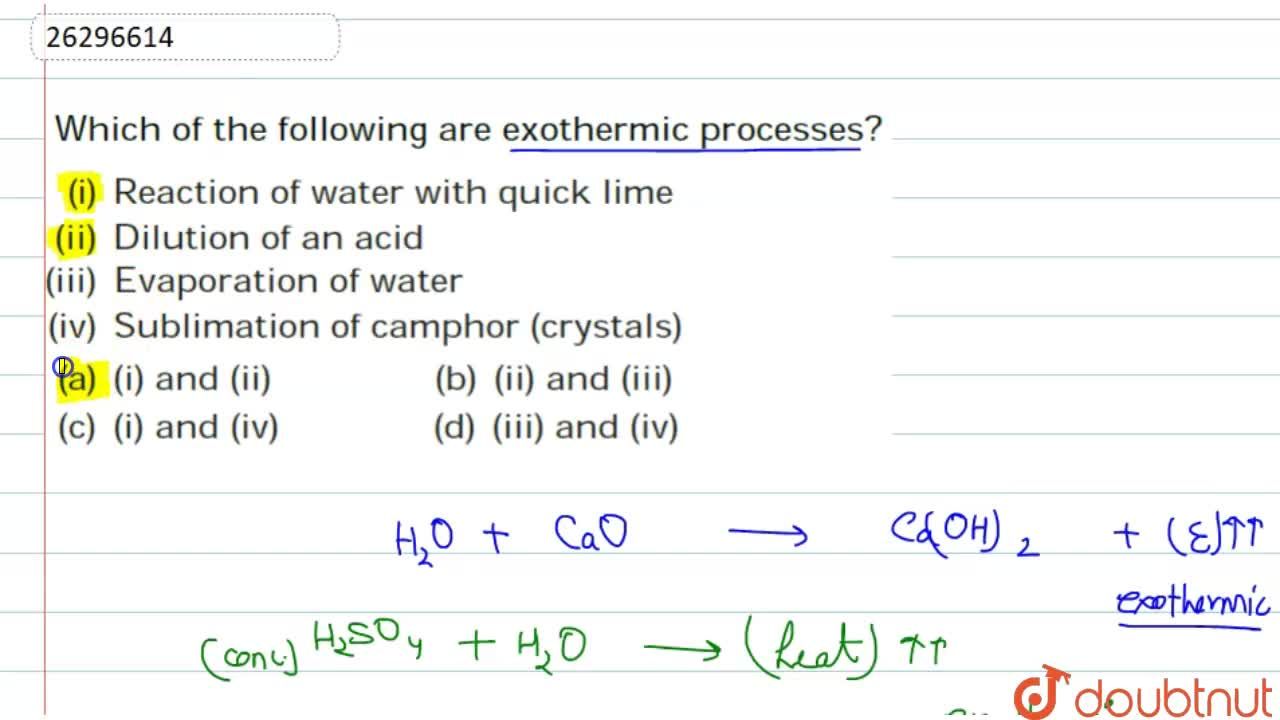 Source: doubtnut.com
Source: doubtnut.com
It is the opposite of an endothermic reaction. An endothermic process requires the absorption of. 1) oxidation of iron involves reaction of elemental iron with oxygen to form iron oxide (rust).
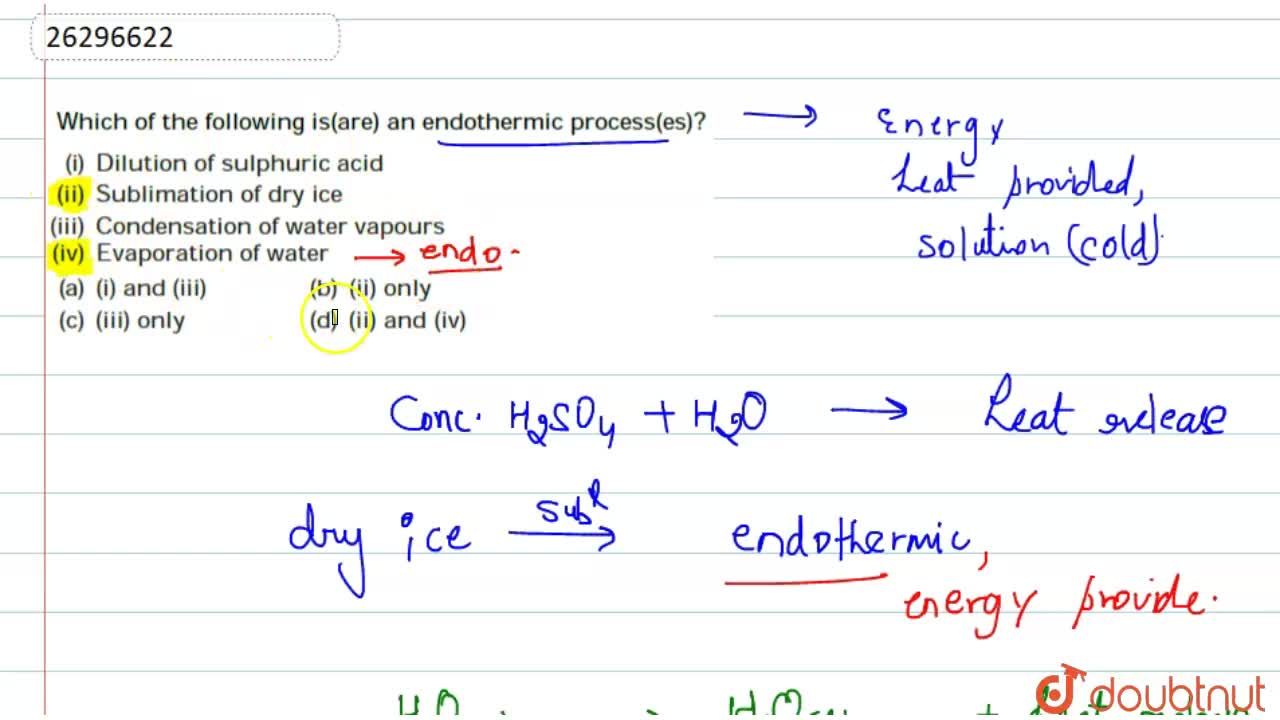 Source: doubtnut.com
Source: doubtnut.com
Quick lime reacts vigorously with water releasing a. Which of the following statements is true? An exothermic reaction is a reaction that releases heat.
 Source: youtube.com
Source: youtube.com
Thus in an exothermic reaction, energy is transferred into the surroundings rather than taking energy from the surroundings as in an endothermic reaction. Δh for an exothermic reaction is positive.c. So, this reaction is an exothermic reaction.
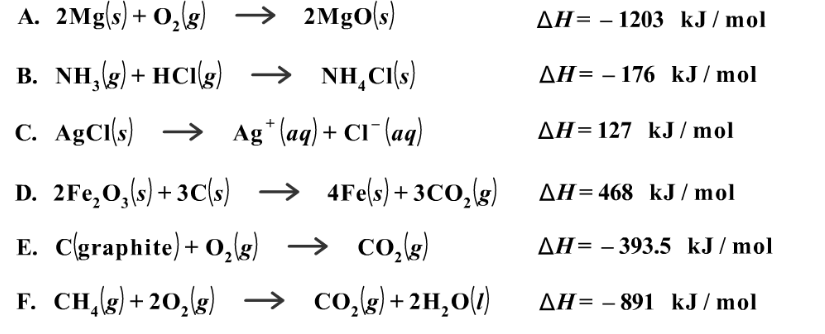 Source: clutchprep.com
Source: clutchprep.com
It is neither endothermic nor exothermic. Which of the following is true of the reaction: What is an exothermic reaction?
 Source: toppr.com
Source: toppr.com
C.)the third intermediate reaction is endothermic. B.)there are two intermediate reactions in this system. B photosynthesis is an exothermic reaction, so.
 Source: thoughtco.com
Source: thoughtco.com
Exothermic reaction means exo meaning releases and thermic means heat. C.)the third intermediate reaction is endothermic. Expressed in a chemical equation:

Exothermic reaction means exo meaning releases and thermic means heat. In this worksheet, we will practice identifying exothermic and endothermic chemical reactions. The reaction in which heat is absorbed is called an endothermic reaction.
 Source: youtube.com
Source: youtube.com
An endothermic process requires the absorption of. Exothermic reaction means exo meaning releases and thermic means heat. A few examples are neutralization, burning a substance, reactions of fuels, deposition of dry ice, respiration, solution of sulfuric acid into water and much more.
 Source: youtube.com
Source: youtube.com
The evaporation of water is an exothermic process.f. Example of a temperature change that might occur in an endothermic reaction. The reaction in which heat is absorbed is called an endothermic reaction.
 Source: youtube.com
Source: youtube.com
An exothermic reaction is a reaction in which energy is released in the form of light or heat. The following reactions are exothermic in an exothermic reaction, heat has been released to the surroundings from the system. In an exothermic reaction, change in enthalpy ( δh) will be negative exothermic reactions.
Also Read :