Which alcohol reacts fastest and by what mechanism? (a) tertiary alcohol by s n 2 (b) secondary alcohol by s n 1 (c) tertiary alcohol by s n 1 (d) secondary alcohol by s n 2.
Which Of The Following Is A Tertiary Alcohol. If it has two r groups, it is a secondary alcohol, and if it has three r groups, it is a tertiary alcohol. But it is easily recognized because oh is close to an x in the molecular structure. Become a study.com member to unlock this answer! (b) when a grignard reagent reacts with a ketone, the addition product is a secondaryalcoho(c) when a grignard reagent reacts with a aldehyde, the addition product is a tertiary alcohol.
 Alcohols & Alkanols | Functional Groups & Structures - Video & Lesson Transcript | Study.com From study.com
Alcohols & Alkanols | Functional Groups & Structures - Video & Lesson Transcript | Study.com From study.com
Related Post Alcohols & Alkanols | Functional Groups & Structures - Video & Lesson Transcript | Study.com :
Which of the following compounds on reaction with. If the hydroxyl carbon only has a single r group, it is known as primary alcohol. Askedjul 24, 2019in chemistryby anshusingh(23.7kpoints) which of the following is a tertiary alcohol. Functional group is bonde to a tertiary allylic carbon.
In a tertiary allylic alcohol the.
It is a tertiary alcohol. Its formula continues to be roh, like other alcohols; Alcohols are organic molecules containing a hydroxyl functional group connected to an alkyl or aryl group (roh). If it has two r groups, it is a secondary alcohol, and if it has three r groups, it is a tertiary alcohol. C) (ch3)2cohch3 is tertiary alcohol. Is pentan 1 ol a tertiary alcohol?
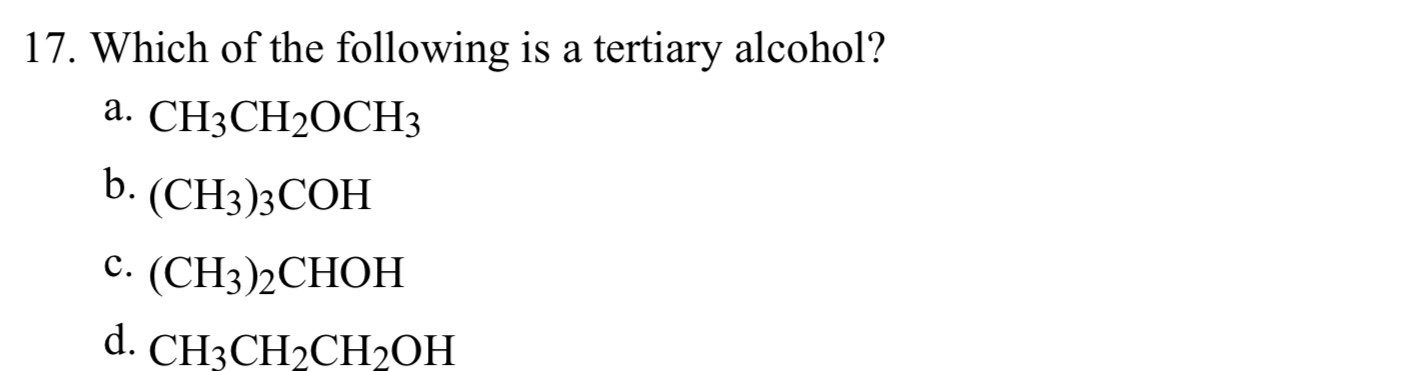 Source: chegg.com
Source: chegg.com
Functional group is bonde to a tertiary allylic carbon. Which of the following is dihydric alcohol [dce 2004] a). The correct option is d) (ch3 3.

The oxidation of alcohols is an important reaction in organic chemistry. Question_answer17) the characteristic grouping of secondary alcohols is [dpmt 1984] a). Tertiary alcohols on reaction with kmno4 at elevated temperature form (a) aldehyde (b) ketone (c) mixture of carboxylic acids containing lesser number of carbon atoms (d) mixture of carboxylic acids containing more number of carbon atoms
A tertiary alcohol is a compound in which a hydroxy group, ‒oh, is attached to a saturated carbon atom which has three other carbon atoms attached to it./span> is ch3ch2ch2oh a tertiary alcohol? What happens when a tertiary alcohol is oxidized? Please check these options because the valency of most of the carbon atoms is not satisfied.
 Source: study.com
Source: study.com
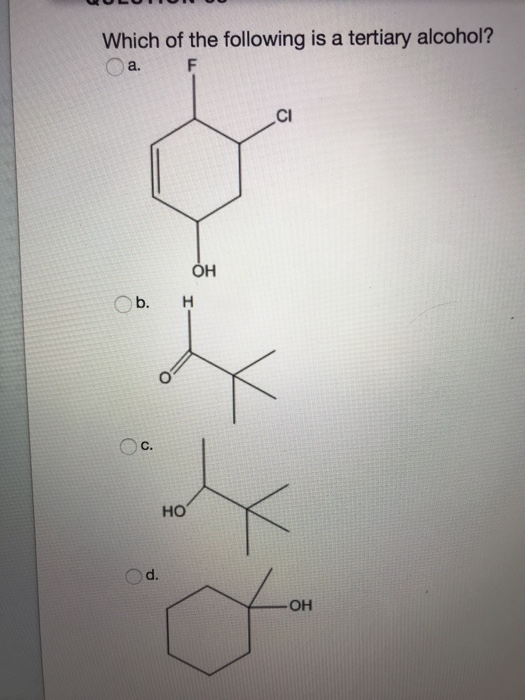
It is a tertiary alcohol. If the hydroxyl carbon only has a single r group, it is known as primary alcohol. (a) when a grignard reagent reacts with a ketone, the addition product is a primary alcohol.
 Source: chegg.com
Source: chegg.com
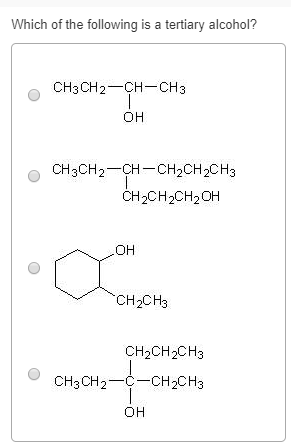
Which of the following compounds on reaction with. If it has two r groups, it is a secondary alcohol, and if it has three r groups, it is a tertiary alcohol. The type of alcohol can also be determined by counting the number of hydrogen atoms bonded to the carbon with the oh group.
 Source: toppr.com
Source: toppr.com
B) alcohols undergo nucleophilic substitution. Tertiary alcohols on reaction with kmno4 at elevated temperature form (a) aldehyde (b) ketone (c) mixture of carboxylic acids containing lesser number of carbon atoms (d) mixture of carboxylic acids containing more number of carbon atoms Will give a tertiary alcohol?

If it has two r groups, it is a secondary alcohol, and if it has three r groups, it is a tertiary alcohol. Hexylresorcinol is listed as an active ingredient in some mouthwashes and throat lozenges. Alcohols are organic molecules containing a hydroxyl functional group connected to an alkyl or aryl group (roh).
 Source: chegg.com
Source: chegg.com
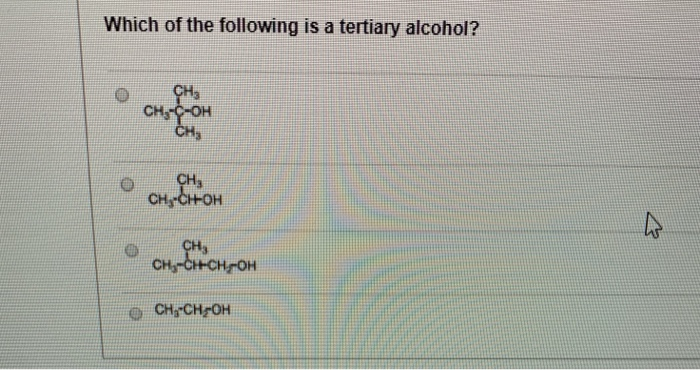
Which of the following compounds on reaction with. Which alcohol reacts fastest and by what mechanism? A) tertiary alcohols have lower boiling points than primary alcohols with an equivalent molecular weight.
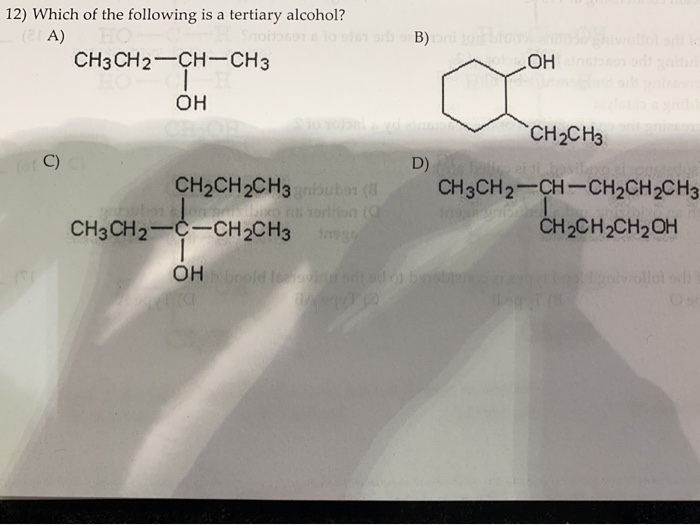 Source: chegg.com
Source: chegg.com
If the hydroxyl carbon only has a single r group, it is known as primary alcohol. Tertiary alcohols on reaction with kmno4 at elevated temperature form (a) aldehyde (b) ketone (c) mixture of carboxylic acids containing lesser number of carbon atoms (d) mixture of carboxylic acids containing more number of carbon atoms If the hydroxyl carbon only has a single r group, it is known as primary alcohol.
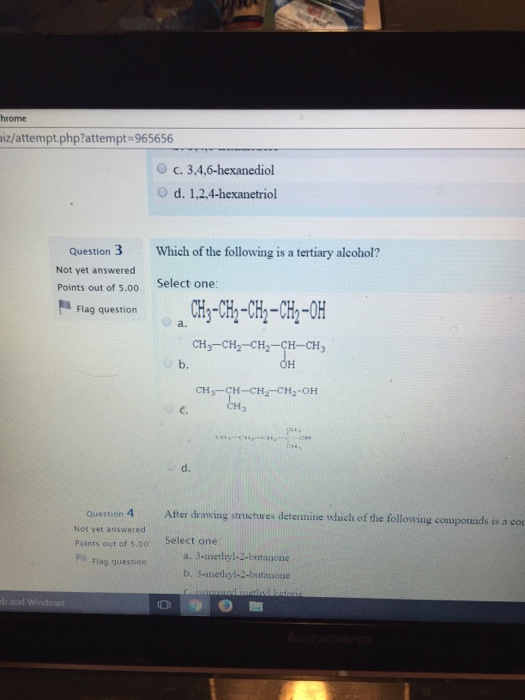
D) tertiary alcohols are metabolised in the body to ketones. Which of the following is a tertiary alcohol? The oxidation of alcohols is an important reaction in organic chemistry.
 Source: chemistrylearner.com
Source: chemistrylearner.com
It is a tertiary alcohol. C) tertiary alcohols undergo dehydration more readily than primary alcohols. ∙tertiary carbocations formed in dehydration reaction are more stable and hence they react readily than primary alcohols.
![Ch3 - Ch = Ch2 [ 2 Nabh4/Oh^- ]1 Hg(Oac)2/Thf/H2Och3 - Oh|Ch - Ch3 Ch3 - Ch = Ch2 [ 2 Nabh4/Oh^- ]1 Hg(Oac)2/Thf/H2Och3 - Oh|Ch - Ch3](https://d2rrqu68q7r435.cloudfront.net/images/5713255/be8ca398-b220-4805-b7b3-da25c81186fc.jpg) Source: toppr.com
Source: toppr.com
Askedjul 24, 2019in chemistryby anshusingh(23.7kpoints) which of the following is a tertiary alcohol. Remember that in primary alcohol, the carbon atom bearing hydroxyl group is attached to only one other carbon atom. But it is easily recognized because oh is close to an x in the molecular structure.
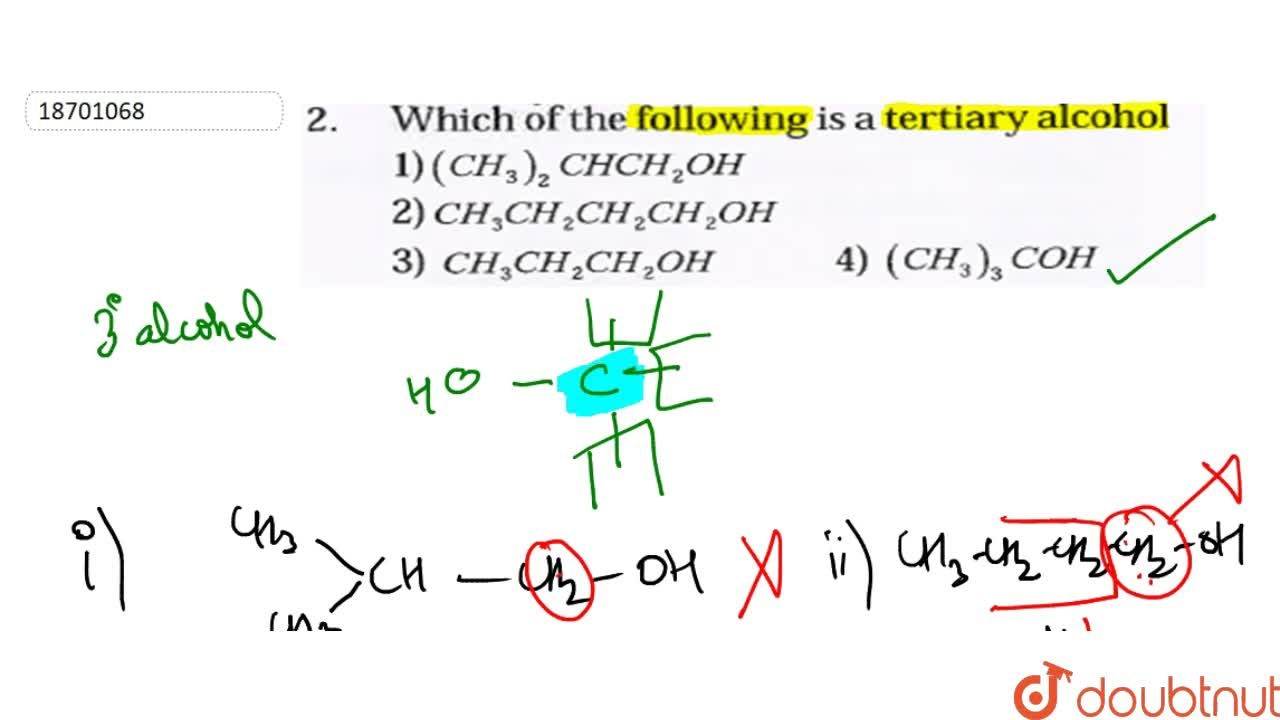 Source: doubtnut.com
Source: doubtnut.com
(a) hcho (b) r 2 co (c) rcn (d) rcocl. If it has two r groups, it is a secondary alcohol, and if it has three r groups, it is a tertiary alcohol. A) tertiary alcohols have lower boiling points than primary alcohols with an equivalent molecular weight.
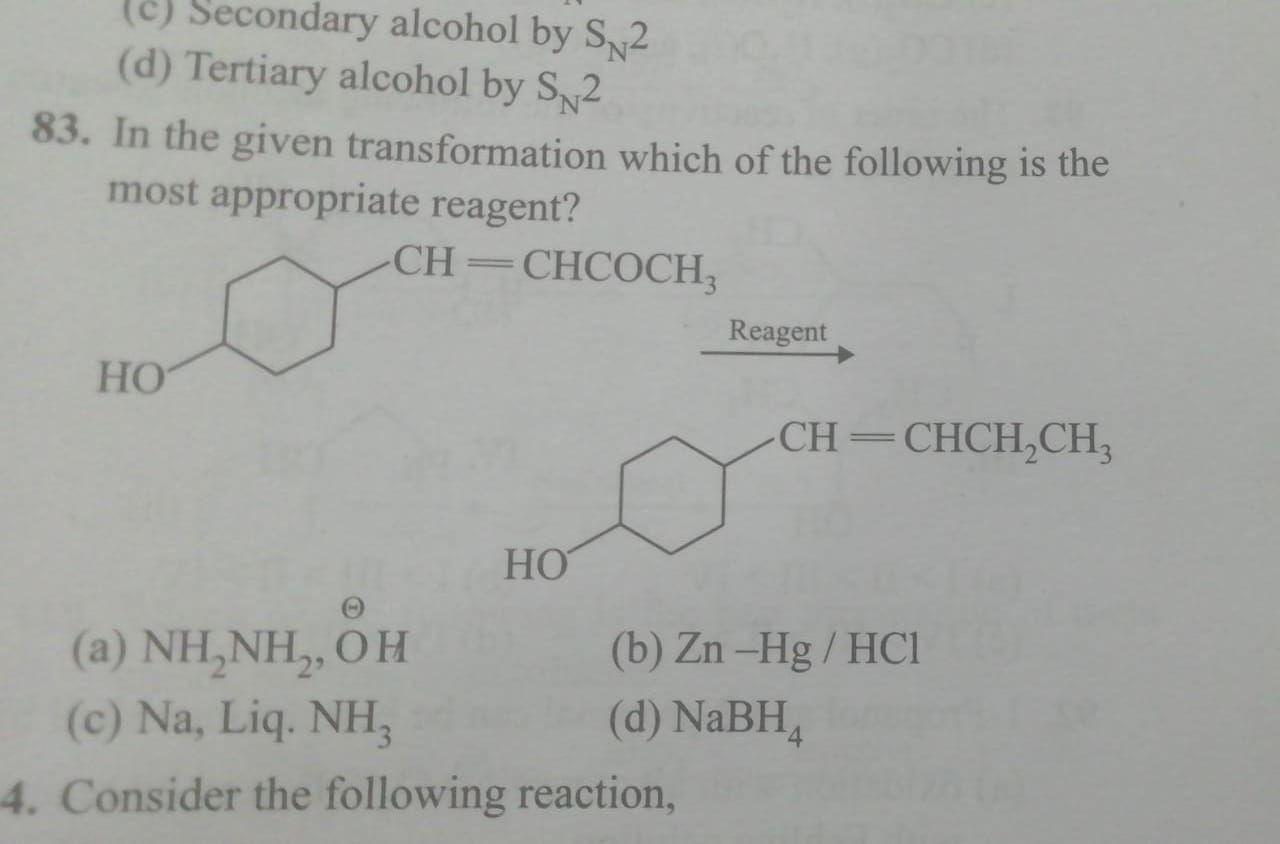 Source: questions-in.kunduz.com
Source: questions-in.kunduz.com
Will give a tertiary alcohol? B) alcohols undergo nucleophilic substitution. A tertiary alcohol is a compound in which a hydroxy group, ‒oh, is attached to a saturated carbon atom which has three other carbon atoms attached to it./span> is ch3ch2ch2oh a tertiary alcohol?
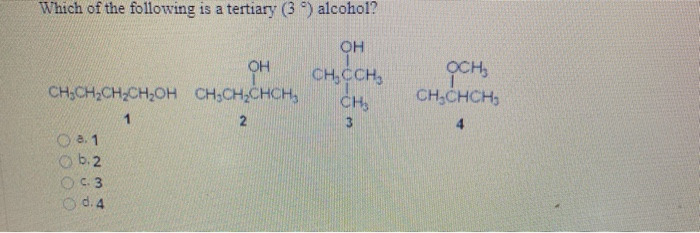 Source: chegg.com
Source: chegg.com
A) tertiary alcohols have lower boiling points than primary alcohols with an equivalent molecular weight. Functional group is bonde to a tertiary allylic carbon. (a) hcho (b) r 2 co (c) rcn (d) rcocl.
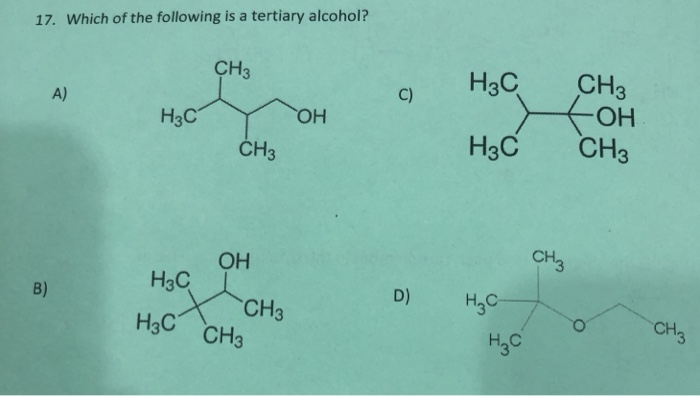 Source: chegg.com
Source: chegg.com
Primary alcohols can be oxidized to form aldehydes and carboxylic acids; (a) tertiary alcohol by s n 2 (b) secondary alcohol by s n 1 (c) tertiary alcohol by s n 1 (d) secondary alcohol by s n 2. Ethyl alcohol can be prepared from grignard reagent by the reaction of :
 Source: chegg.com
Source: chegg.com
If it has two r groups, it is a secondary alcohol, and if it has three r groups, it is a tertiary alcohol. An unknown alcohol is treated with “lucas reagent” to determine whether the alcohol is primary, secondary or tertiary. A tertiary alcohol is one in which the hydroxyl group, oh, is attached to a tertiary carbon.
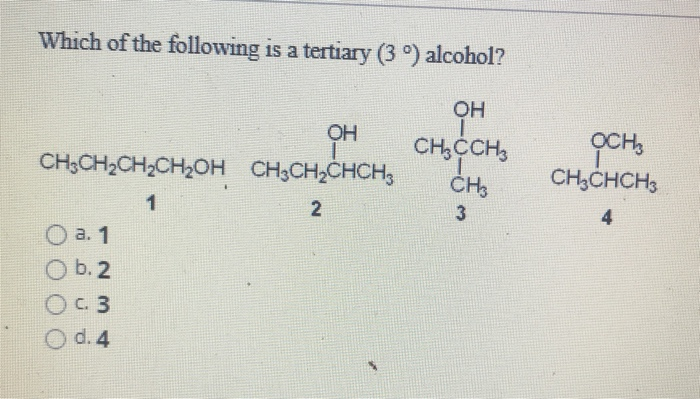 Source: chegg.com
Source: chegg.com
Neopentyl alcohol is primary alcohol as o h attached to the c which is attached to only one c. The correct option is d) (ch3 3. Neopentyl alcohol is primary alcohol as o h attached to the c which is attached to only one c.
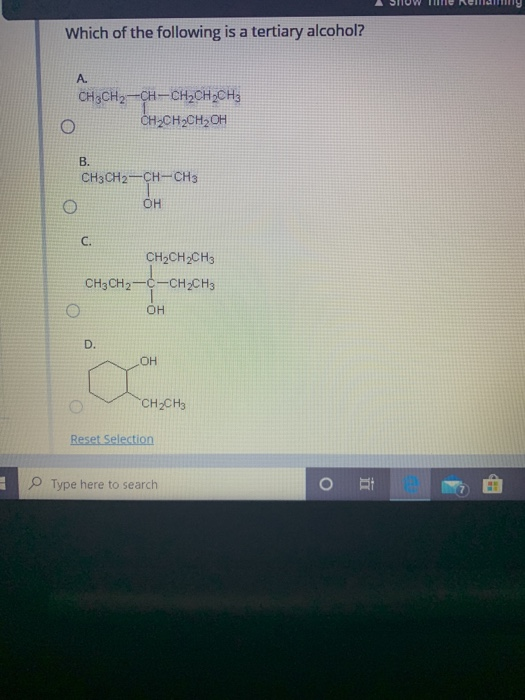 Source: chegg.com
Source: chegg.com
Functional group is bonde to a tertiary allylic carbon. Its formula continues to be roh, like other alcohols; In tertiary alcohol, the carbon atom bearing hydroxyl group is attached to three other carbon atoms.
 Ch_(3)-Overset(Ch_(3))O… - Youtube")
Source: youtube.com
If it has two r groups, it is a secondary alcohol, and if it has three r groups, it is a tertiary alcohol. ∙tertiary carbocations formed in dehydration reaction are more stable and hence they react readily than primary alcohols. Will give a tertiary alcohol?
Also Read :






