Acid pk a ha 4.00 hb 7.00 hc 10.00 hd 11.00 a. A) always the component in the greatest amount b) the dissolving.
Which Of The Following Is A Solution. Based on the information in the graph, which of the following is the best prediction of how many grams of the compound will dissolve at 40 c? Which of the following is an example of a solid solution? All will have the same ph because the concentrations are the same. Which of the following are ‘pure substances’?
 Which Of The Following Is A Solution Of The Equation X+2Y=7 - Brainly.in From brainly.in
Which Of The Following Is A Solution Of The Equation X+2Y=7 - Brainly.in From brainly.in
Related Post Which Of The Following Is A Solution Of The Equation X+2Y=7 - Brainly.in :
Industries create four types of pollution mainly air, water, land and noise. A) a solution of acetic acid and sodium acetate b) a solution of hydrochloric acid and sodium acetate c) a solution of sulfuric acid and sodium sulfate d) a solution of acetic acid and sodium sulfate e) a. Which of the following are ‘pure substances’? Of the compound will dissolve in 100 ml of water at 40 c.
(b) solution like y are known as saturated solution.
(c) if solution y at 30 o c is cool down to 10 o c by keeping the beaker in crushed ice, then some of the dissolved solid will separate out from the solution and settle at the bottom of the beaker as crystals. A compound containing carbon, hydrogen and oxygen d) dental fillings: They cannot be broken into anything else by physical or chemical means. An amalgam e) 80 proof vodka: Any species that can produce hydroxide ions in aqueous solution ____ 5. Ice, milk, iron, hydrochloric acid, calcium oxide, mercury, brick, wood, air.
 Source: youtube.com
Source: youtube.com
E) the kw is independent of what the solution contains. Rushing the speaker and making him feel that he’s wasting the listener’s time. (iv) briefly explain the indirect sources of information of the interior of the earth other than those of seismic activity.
 Source: chegg.com
Source: chegg.com
In this case, salt water is a solution because it involves salt that is dissolved in water. C) the solution is basic. (a) solution like x are known as unsaturated solution.
(0,… - Youtube")
Source: youtube.com
Solve the equation and explain why it has an infinite number of solutions. The correct answer is a. 10 ml of (m 10) hcl and 90 ml of.
 Source: chegg.com
Source: chegg.com
E) the kw is independent of what the solution contains. Not responding to the speaker’s requests. Earthquake wave develop shadow zone because p and s waves follow a curved path inside the earth due to increasing capacity.
 Source: brainly.com
Source: brainly.com
Which of the following compounds is manufactured by using the chemical effect of electric current? Ice, milk, iron, hydrochloric acid, calcium oxide, mercury, brick, wood, air. Sulphur (ii) pure substances which are made up of same kind of molecules.
 Source: toppr.com
Source: toppr.com
A disolved solute will not settle out or separate from the solvent in a solution. We can substitute the values in the given equation and can check whether lhs is equal to rhs or not. 82, which of the following is a buffer solution?

The correct answer was given: Hence out of the four options, the correct option is (d) 4x + 7 = x + 2, which is neither a fraction nor an integer When a solution is saturated and additional solute sits at the bottom of the vessel:
 Source: study.com
Source: study.com
(b) solution like y are known as saturated solution. We can substitute the values in the given equation and can check whether lhs is equal to rhs or not. Solve the equation and explain why it has an infinite number of solutions.
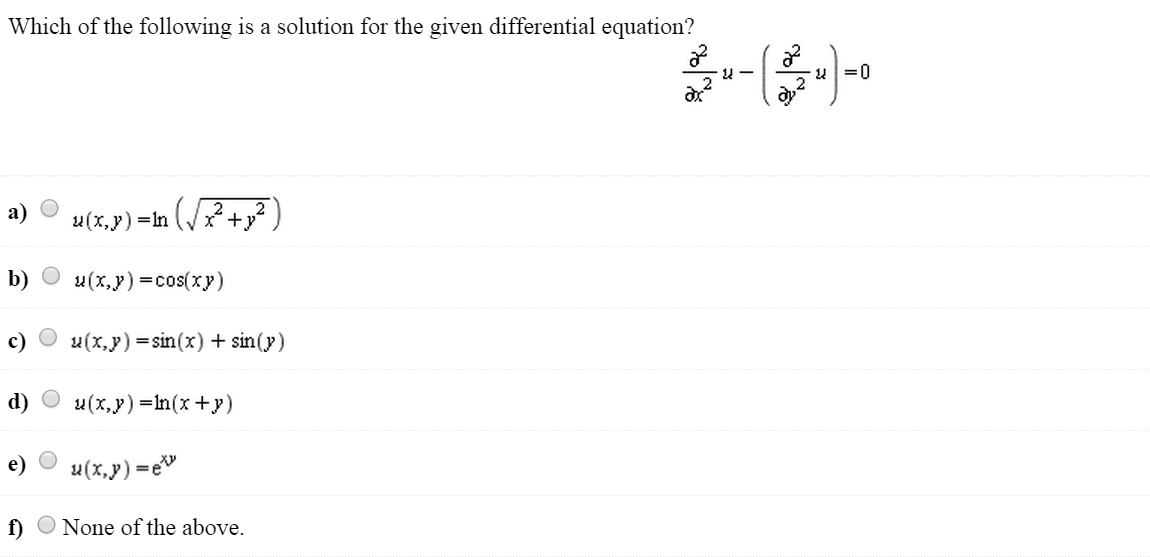 Source: chegg.com
Source: chegg.com
Showing interest in something other than the conversation. (a) ammonium hydroxide (b) sodium carbonate (c) magnesium hydroxide (d) sodium hydroxide. Hence out of the four options, the correct option is (d) 4x + 7 = x + 2, which is neither a fraction nor an integer
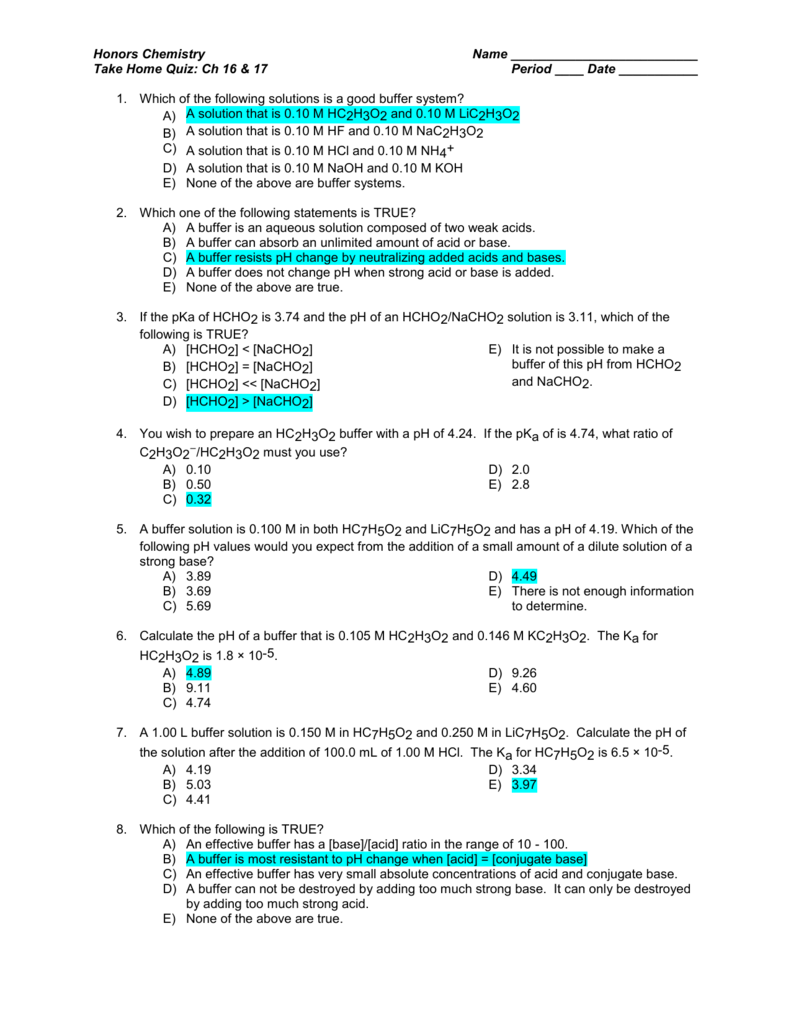 Source: studylib.net
Source: studylib.net
Which of the following statements best describes the phrase like dissolves like? (i) pure substances which are made up of same kind of atoms. > a buffer solution (ph buffer or hydrogen ion buffer, more precisely) is an aqueous solution composed of a combination or a mixture of a neutral or a very weak acid and its conjugate base, or vice versa.
 Source: brainly.in
Source: brainly.in
Which of the following are defining traits of solution? To choose the correct option. > a buffer solution (ph buffer or hydrogen ion buffer, more precisely) is an aqueous solution composed of a combination or a mixture of a neutral or a very weak acid and its conjugate base, or vice versa.
 Source: youtube.com
Source: youtube.com
Which of the following are ‘pure substances’? Earthquake wave develop shadow zone because p and s waves follow a curved path inside the earth due to increasing capacity. (a) car bumper (b) gas stove (c) frying pan (d) bicycle bell.
 Source: brainly.com
Source: brainly.com
100 ml of (m 10) hcl and 100 ml of (m 10) naoh. Which of the following are defining traits of solution? Option (d) is the answer.
 Source: youtube.com
Source: youtube.com
Here the given equation is Further, we at shaalaa.com provide such solutions so that students can prepare for written exams. A milk is solution of different components.
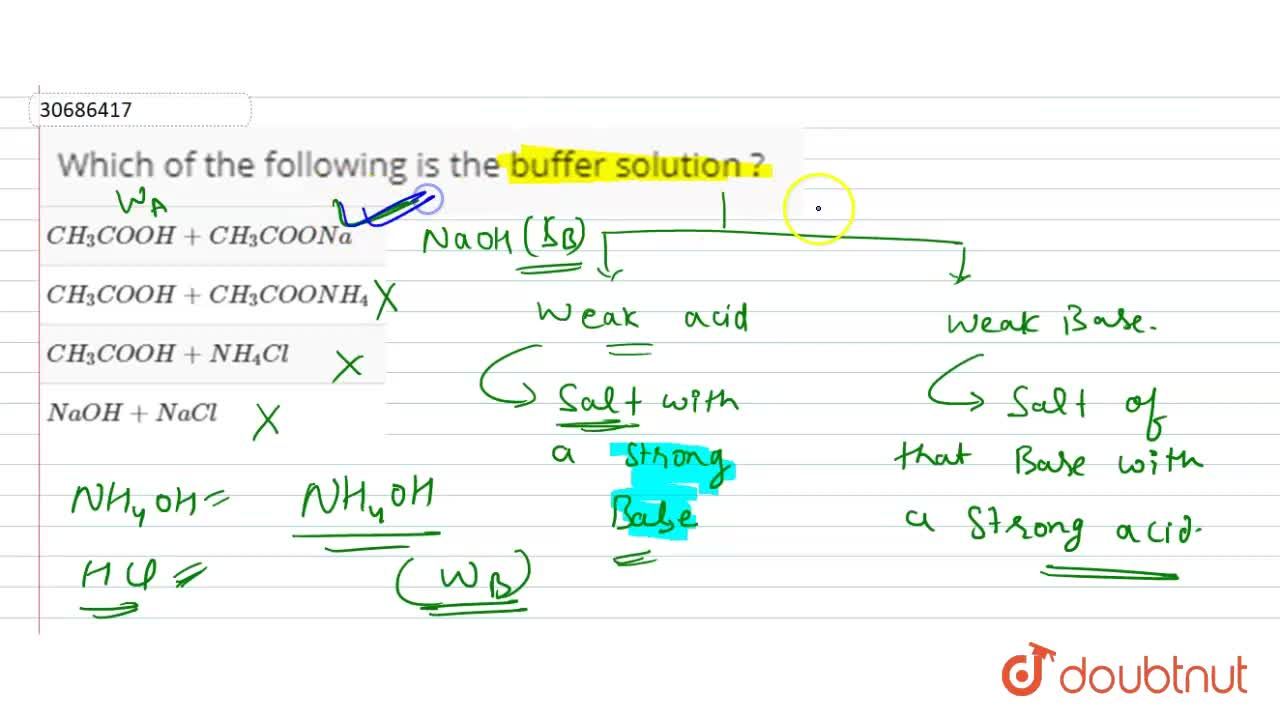 Source: doubtnut.com
Source: doubtnut.com
Showing interest in something other than the conversation. Limestone, silica, alumina, and gypsum are important raw materials used in the manufacturing of cement. Industries create four types of pollution mainly air, water, land and noise.

Ice, milk, iron, hydrochloric acid, calcium oxide, mercury, brick, wood, air. Which one of the following statements. Which of the following solutions is a buffer?
 Source: chegg.com
Source: chegg.com
Which of the following compounds is manufactured by using the chemical effect of electric current? The correct answer was given: A solution can only occur in water b.
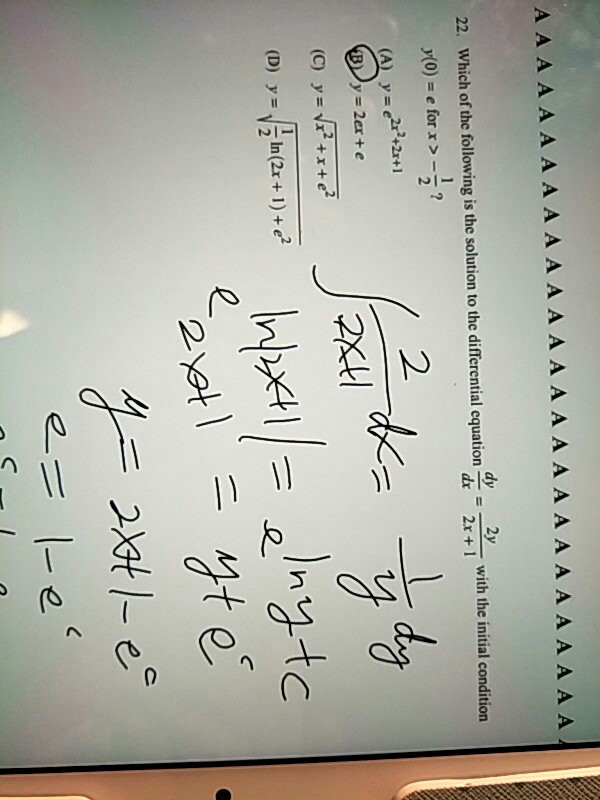 Source: chegg.com
Source: chegg.com
Shaalaa.com has the cbse class 10 chemistry (science) solutions in a manner that help students grasp basic concepts better and faster. (b) solution like y are known as saturated solution. (iv) briefly explain the indirect sources of information of the interior of the earth other than those of seismic activity.
 Source: youtube.com
Source: youtube.com
Which of the four solutions will have the lowest ph and be most acidic? A solvent and a solute with similar attractive forces will easily make a solution. In this case, salt water is a solution because it involves salt that is dissolved in water.
 Source: chegg.com
Source: chegg.com
Which of the following equations has an infinite number of solutions? Any species that can produce hydroxide ions in aqueous solution ____ 5. Which of the following is not considered a bad.
Also Read :





