Here, no change of oxidation state takes place. Of c increases from +2 (in co) to +4 (in c0 2) and therefore, co acts as a reducing agent.
Which Of The Following Is A Redox Reaction. If there is no change in oxidation number, then the reaction is not a redox reaction. B) the products are the constituent elements. Reduction is when an atom gains an electron or electrons. A chemical substance is said to be oxidized when it loses electrons or the net positive charge on that species increases and a chemical substance is said to be reduced when it gains electrons or the net negative charge on that species increases.

Related Post Solved Which One Of The Following Is Not Redox Reaction? | Chegg.com :
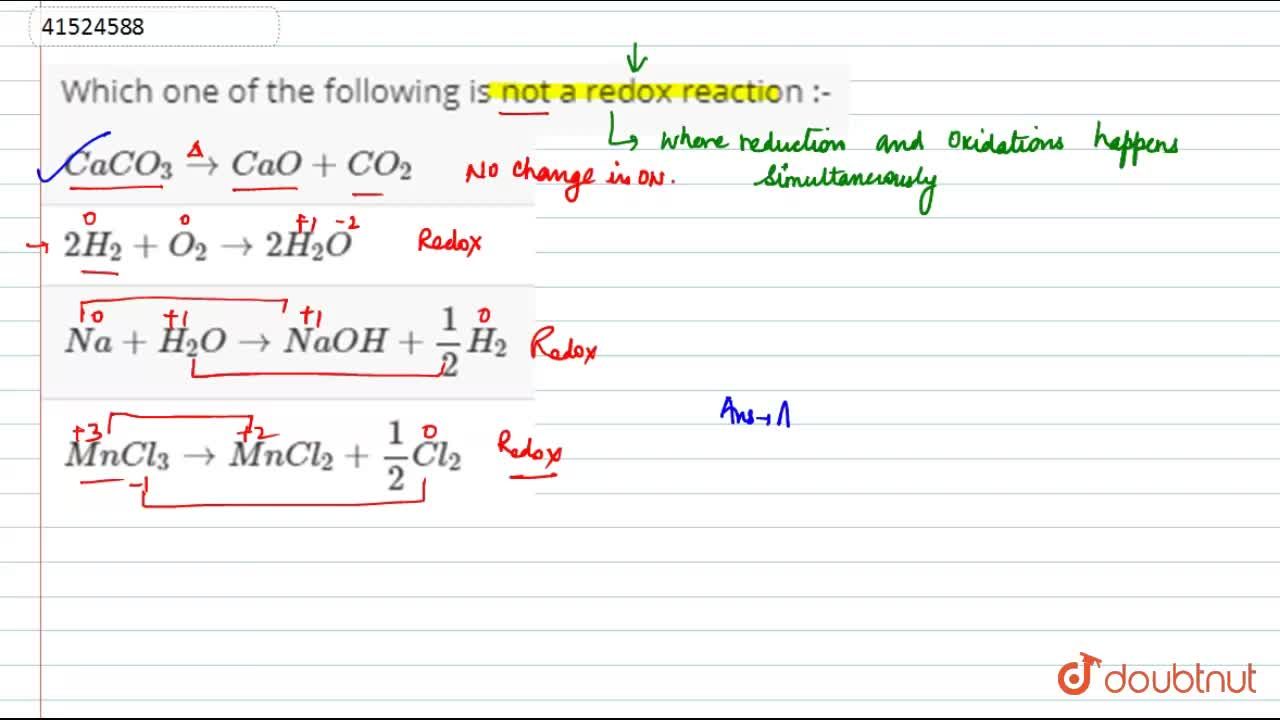
Therefore, it is not a redox reaction. Thus, reaction (i) is a redox reaction. $cac{o_3} \to cao + c{o_2}$ is a decomposition reaction. A chemical substance is said to be oxidized when it loses electrons or the net positive charge on that species increases and a chemical substance is said to be reduced when it gains electrons or the net negative charge on that species increases.
$cac{o_3} \to cao + c{o_2}$ is a decomposition reaction.
(a) evaporation of h 2 o (b) both oxidation and reduction (c) h 2 so 4 with naoh (d) in atmosphere o 3 from o 2 by lightning A redox reaction is defined as a chemical reaction that involves both oxidation and reduction. These include combustion, rusting, photosynthesis, respiration and decomposition. Cu0 + 2agno3+1 → cu(no3)2+2 + 2ag0this reaction is an example of redox reaction in which cu is being oxidised to cu2+ while ag+ is getting reduced to ag. Gain of electron or decrease in oxidation state. Thus, this is a redox reaction.
 Source: brainly.in
Source: brainly.in
I�m jewish nice plush toys and it�s called sale museum this year. The substance that is reduced because it gains electrons Many processes that occur around us are redox reactions.
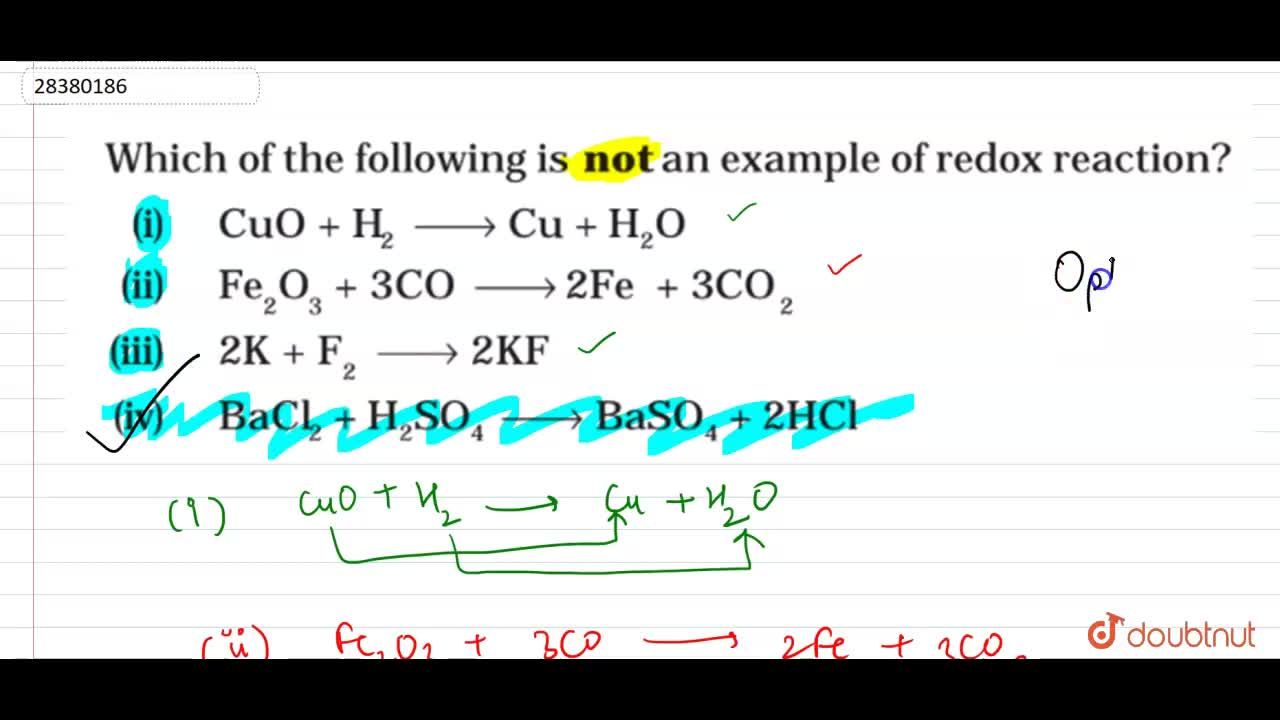 Source: doubtnut.com
Source: doubtnut.com
A redox reaction is one in which there is a change in the oxidation number of the reactants from left to right of the reaction equation. Even so in this question they asked which of the following is a redox reaction and scl plus cannot produce an and meticulous case here. Which of the following is true about a redox reaction?
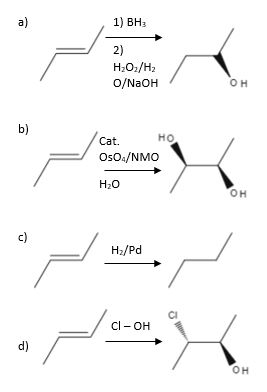 Source: study.com
Source: study.com
Which of the following reactions is a redox reaction? We will check one by one each reaction by calculating the oxidation number of all species. A redox reaction is a type of reaction which involves transfer of electrons among two species.
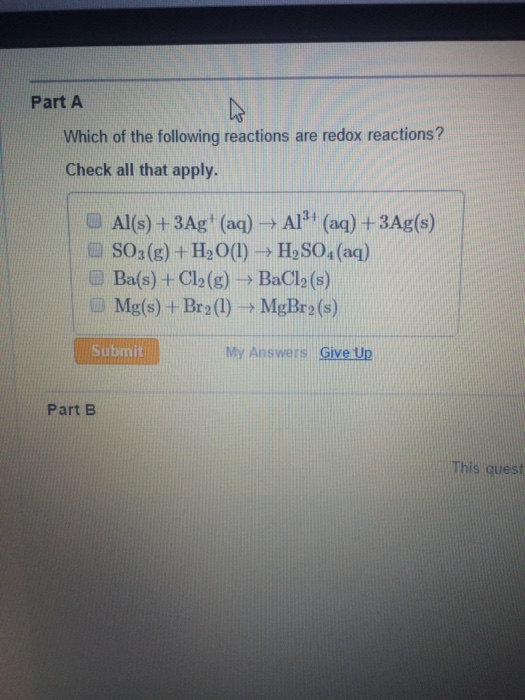
Pb₂²+ + 2br⁻ → pbbr c. A redox reaction is a type of reaction which involves transfer of electrons among two species. Cu0 + 2agno3+1 → cu(no3)2+2 + 2ag0this reaction is an example of redox reaction in which cu is being oxidised to cu2+ while ag+ is getting reduced to ag.
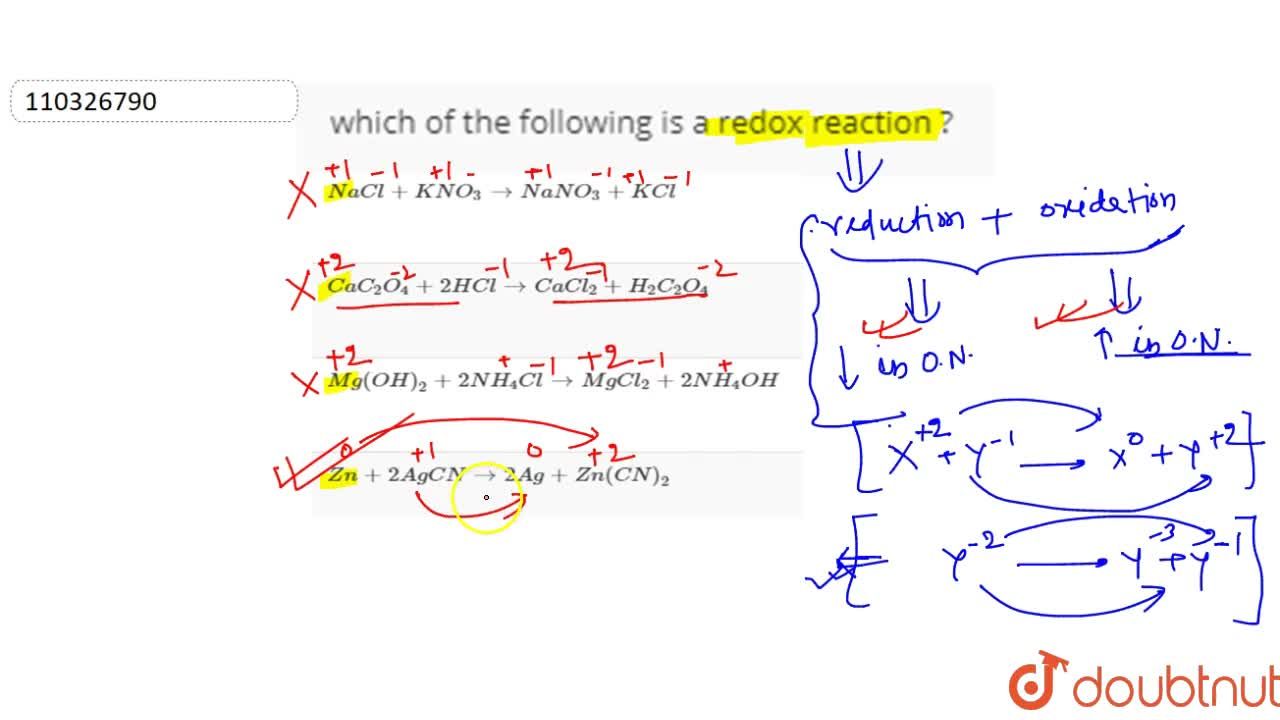 Source: doubtnut.com
Source: doubtnut.com
In a redox reaction, an electron is lost by the reducing agent. Manufacturing of many compounds like caustic soda is also a redox process and corrosion of metal is also a redox process. You see english choices, you place currency and to so in this case,.
 Source: toppr.com
Source: toppr.com
I�m jewish nice plush toys and it�s called sale museum this year. We have two waters reacting with each other, forming two hydrogen and two oxygen�s using the oxidation of the real oxygen minus two. Of none of the atoms undergo a change and therefore, this is not a redox reaction.
![Which Of The Following Reactions Is A Redox Reaction? (A).Cuso_(4)+4Nh_(3) To [Cu(Nh_(3))_(4)]So_(4 - Youtube](https://i.ytimg.com/vi/N6TEEBGFyzc/maxresdefault.jpg "Which Of The Following Reactions Is A Redox Reaction? (A).Cuso_(4)+4Nh_(3) To [Cu(Nh_(3))_(4)]So_(4 - Youtube")
Source: youtube.com
All the processes given above are redox processes as in electrochemical process like extraction of highly reactive metals is a redox reaction. Pb₂²+ + 2br⁻ → pbbr c. So you csn plus esposito four.
 Source: doubtnut.com
Source: doubtnut.com
We will check one by one each reaction by calculating the oxidation number of all species. Loss of electron or increase in oxidation state. Cu + s → cus
 Source: chegg.com
Source: chegg.com
Pb₂²+ + 2br⁻ → pbbr c. A chemical substance is said to be oxidized when it loses electrons or the net positive charge on that species increases and a chemical substance is said to be reduced when it gains electrons or the net negative charge on that species increases. Of c increases from +2 (in co) to +4 (in c0 2) and therefore, co acts as a reducing agent.
 Source: chegg.com
Source: chegg.com
Oxidation number denotes the oxidation state of an element in a compound ascertained according to a set of rules formulated on the basis that electron pair in a covalent bond belongs entirely to more electronegative elements. We will check one by one each reaction by calculating the oxidation number of all species. If there is a change in oxidation number, then the reaction is a redox reaction.
 Source: toppr.com
Source: toppr.com
(a) a g + 1 n + 5 o 3 − 2 + k + 1 i − 1 a g + 1 i − 1 + k + 1 n + 5 o 3 − 2. Cu + s → cus Therefore, it is not a redox reaction.
 Source: neetlab.com
Source: neetlab.com
Which of the following reactions is a redox reaction(can be more than one)?show with oxidation numbers. Even so in this question they asked which of the following is a redox reaction and scl plus cannot produce an and meticulous case here. Manufacturing of many compounds like caustic soda is also a redox process and corrosion of metal is also a redox process.
 Source: youtube.com
Source: youtube.com
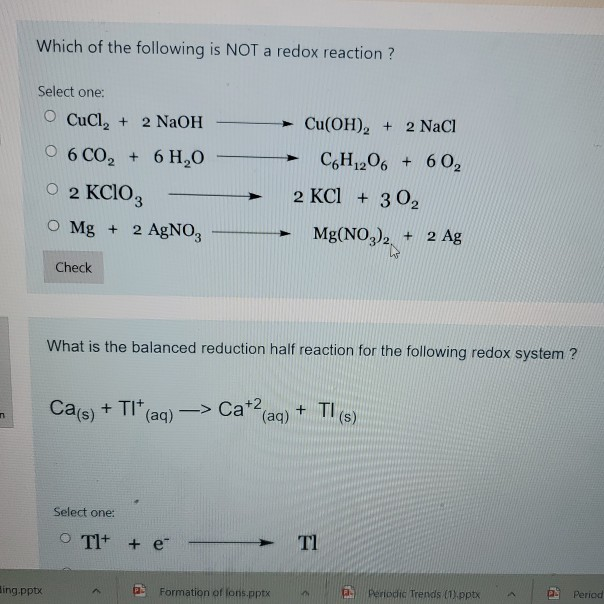
Which of the following is redox reaction? Gain of electron or decrease in oxidation state. A) 2 na + 2 h2o à 2 naoh + h2 / c) 2 k + f2 à 2 kf b) mgbr2 + 2 naf à mgf2 + 2 nabr / d) ch4 + 2 o2 à co2 + 2 h2o.
 Source: numerade.com
Source: numerade.com
Cu + s → cus Here, no change of oxidation state takes place. Redox reaction is that reaction in which both oxidation and reduction of a chemical species takes place at a time.
 Source: oneclass.com
Source: oneclass.com
Of c increases from +2 (in co) to +4 (in c0 2) and therefore, co acts as a reducing agent. Then when we go to there are mental forms. Of c increases from +2 (in co) to +4 (in c0 2) and therefore, co acts as a reducing agent.
 Source: homeworklib.com
Source: homeworklib.com
Two plush toys and forage. Therefore, it is not a redox reaction. A) the products are unpredictable.

Thus, reaction (i) is a redox reaction. So the correct answer is option (d). Therefore, it is not a redox reaction.
 Source: zenius.net
Source: zenius.net
Two plush toys and forage. (a) evaporation of h 2 o (b) both oxidation and reduction (c) h 2 so 4 with naoh (d) in atmosphere o 3 from o 2 by lightning So, also a change in oxidation number there, for this is a redox reaction reaction.

Of none of the atoms undergo a change and therefore, this is not a redox reaction. We can find out the redox reaction if there has been a transfer of electrons means there should be change in the oxidation number of the reactants and the products. So you csn plus esposito four.
 Source: toppr.com
Source: toppr.com
Of c increases from +2 (in co) to +4 (in c0 2) and therefore, co acts as a reducing agent. The substance that is reduced because it gains electrons You see english choices, you place currency and to so in this case,.
Also Read :





