Hydrochloric acid, sodium hydroxide, aluminum chloride. Nonelectrolyte solutions a common example of a nonelectrolyte is glucose, or c6h12o6.
Which Of The Following Is A Nonelectrolyte. Identify the substance underlined, in each of the following cases: Kno3, kno2, and hno3 are strong electrolytes; It is ch2o that is a nonelectrolyte in aqueous solution since it does not dissociates into ions when dissolved in water. Write the net ionic equation for the following reaction.
 Answered: Which One Of The Following Is A… | Bartleby From bartleby.com
Answered: Which One Of The Following Is A… | Bartleby From bartleby.com
Related Post Answered: Which One Of The Following Is A… | Bartleby :
Hydrochloric acid, sodium hydroxide, aluminum chloride. A) calcium chloride (cacl2) b) potassium hydroxide (koh) c). C 2 h 6 o. Urea, benzene, sugar, ethanol, chloroform ,.
Which of the following aqueous solutions is a nonelectrolyte?
Glucose, a sugar with the chemical formula c6h12o6, is a typical example of a nonelectrolyte. Urea, benzene, sugar, ethanol, chloroform ,. Kno3, kno2, hno3, hno2, ans: A) naf d) naoh b) hno 3 e) c 6h 12o 6 (glucose) c) ch 3cooh (acetic acid) ans: Write the net ionic equation for the following reaction. Which of the following when dissolved in water will form a nonelectrolyte?

Hydrochloric acid, sodium hydroxide, aluminum chloride. A sweepstakes is a form of sales promotion that offers: Rhomus inc.is a canadian entertainment company that produces and markets tv programs.to generate customer interest for a new program that it is planning to launch, the company asks its target
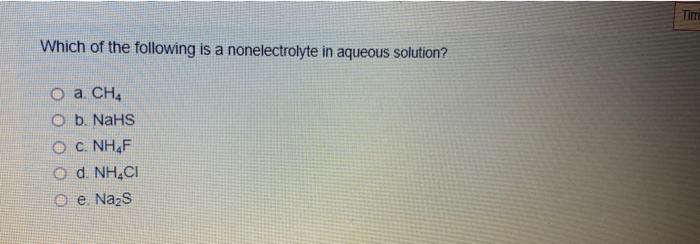
The electrical conductivities of the following 0.100 m solutions were measured in an apparatus that contained a light bulb as. Identify the substance underlined, in each of the following cases: View solution > classify the.

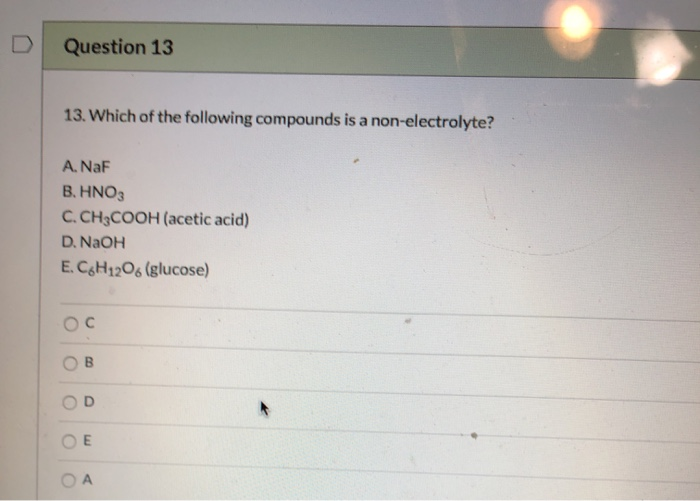
Rhomus inc.is a canadian entertainment company that produces and markets tv programs.to generate customer interest for a new program that it is planning to launch, the company asks its target Kno3, kno2, hno3, hno2, ans: Which of the following compounds is a nonelectrolyte?

Which one of the following compounds is a nonelectrolyte when dissolved in water? A) naoh d) kf b) hno 3 e) ch 3 cooh (acetic acid) c) c 2 h 6 o (ethanol) ans: Identify the following compounds as a strong electrolytes, weak electrolytes, or nonelectrolytes:
 Source: chegg.com
Source: chegg.com
O c6h12o6 (glucose) o naoh o hno3 o naf o ch3cooh (acetic acid) o c6h12o6 (glucose) o naoh o hno3 o naf o ch3cooh (acetic acid) Identify the following compounds as a strong electrolytes, weak electrolytes, or nonelectrolytes: Which of these compounds is a nonelectrolyte?
 Source: brainly.com
Source: brainly.com
Based on the solubility rules, which one of the following compounds should be insoluble in water? Based on the solubility rules, which one of the following compounds should be insoluble in water? It is ch2o that is a nonelectrolyte in aqueous solution since it does not dissociates into ions when dissolved in water.

View solution > classify the. Of the following substances, an aqueous solution of _____ will form basic solutions. A) naf d) naoh b) hno 3 e) c 6h 12o 6 (glucose) c) ch 3cooh (acetic acid) ans:
 Source: chegg.com
Source: chegg.com
Which one of the following compounds is a nonelectrolyte when dissolved in water? Nonelectrolyte solutions a common example of a nonelectrolyte is glucose, or c6h12o6. A) calcium chloride (cacl2) b) potassium hydroxide (koh) c) sodium acetate (nach3co2) d) acetic acid (ch3co2h) e) glucose (c6h12o6)
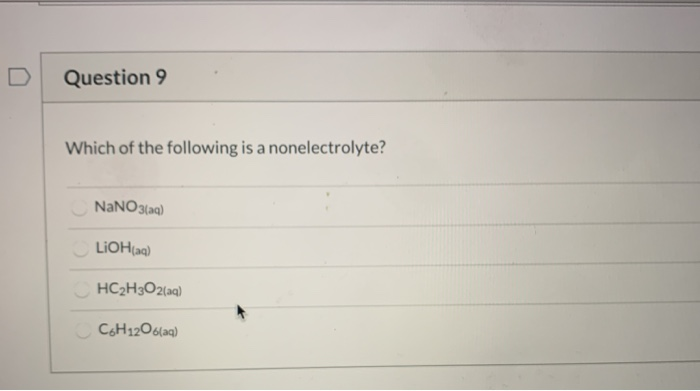 Source: chegg.com
Source: chegg.com
Which of the following compounds is a nonelectrolyte? Hno2 is a weak electrolyte. A) naoh d) kf b) hno 3 e) ch 3cooh (acetic acid) c) c 2h 6o (ethanol)
 Source: bartleby.com
Source: bartleby.com
Nonelectrolytes are usually held together by covalent bonds rather than ionic ones. Which is the name for hno3 in an aqueous solution? View solution > name the particles present in:
 Source: clutchprep.com
Source: clutchprep.com
Kno3, kno2, hno3, hno2, ans: When 20.0 ml of a 0.250 m (nh4)2s solution is added to 150.0 ml of a solution of cu(no3)2, a cus precipitate forms. Which one of the following is a
 Source: clutchprep.com
Source: clutchprep.com
Which of these compounds is a nonelectrolyte? I believe the correct answer from the choices listed above is option d. Hno2 is a weak electrolyte.

Kno3, kno2, and hno3 are strong electrolytes; Rhomus inc.is a canadian entertainment company that produces and markets tv programs.to generate customer interest for a new program that it is planning to launch, the company asks its target The particles present in a liquid such as kerosene, that is a non electrolyte.

Solutions containing glucose do not, therefore. Glucose (sugar) readily dissolves in water, but because it does not dissociate into ions in solution, it is considered a nonelectrolyte; Kno3, kno2, and hno3 are strong electrolytes;
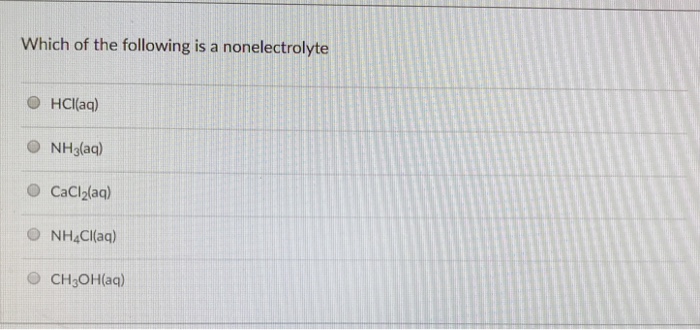
Which of the following compounds is a nonelectrolyte? A) naoh d) kf b) hno 3 e) ch 3 cooh (acetic acid) c) c 2 h 6 o (ethanol) ans: Aqueous iron(iii) sulfate is added to aqueous sodium.
 Source: nagwa.com
Source: nagwa.com
Which of the following compounds is a nonelectrolyte? Which one of the following compounds is a nonelectrolyte when dissolved in water? A) calcium chloride (cacl 2) b) potassium hydroxide (koh) c) sodium acetate (nach 3 co 2) d) acetic acid (ch 3 co 2 h) e) glucose (c 6 h 12 o 6)
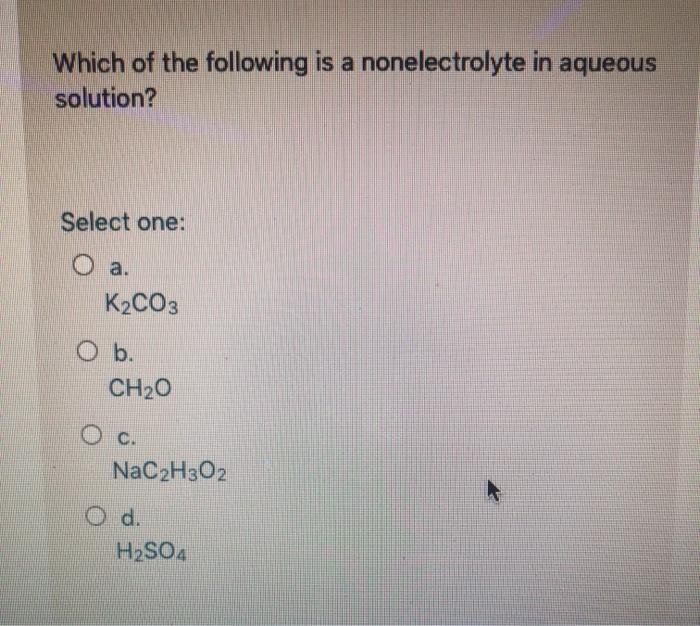
Which one of the following compounds is a nonelectrolyte when dissolved in water? Which aqueous solution has the highest boiling point at standard pressure in water, hno3 produces h+ ions. Which of the following is a nonelectrolyte?
 Source: chegg.com
Source: chegg.com
Which one of the following compounds is a nonelectrolyte when dissolved in water? It is ch2o that is a nonelectrolyte in aqueous solution since it does not dissociates into ions when dissolved in water. Which of the following compounds is a nonelectrolyte?
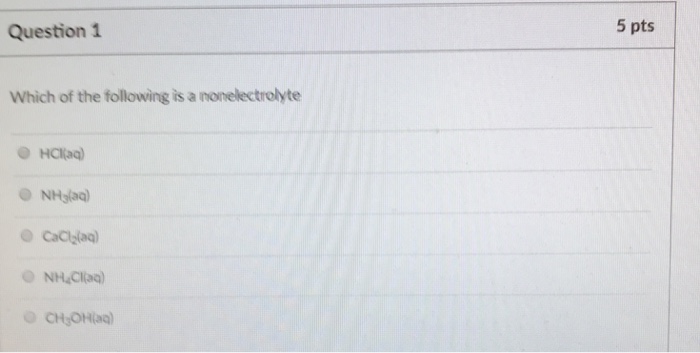
Kno3, kno2, hno3, hno2, ans: A sweepstakes is a form of sales promotion that offers: Based on the solubility rules, which one of the following compounds should be insoluble in water?
Also Read :





