All are lewis acids as they have an incomplete octet. Which one of following is the strongest acid;
Which Of The Following Is A Lewis Acid. According to lewis concept of acids and bases, an acid is a substance which can accept a lone pair of electrons whereas a base is a substance which can donate a lone pair of electrons. Question (16) which of the following is a lewis acid? • it will react with a lewis base. The increasing order of acid strength of the following acid is:
 Solved Which Of The Following Are Lewis Acids And Which Of | Chegg.com From chegg.com
Solved Which Of The Following Are Lewis Acids And Which Of | Chegg.com From chegg.com
Related Post Solved Which Of The Following Are Lewis Acids And Which Of | Chegg.com :
Asked may 20, 2019 in chemistry by fluffynut. It is electron deficient, so it can accept low pair of electron and behave as hewis acid. alcl_(3), sncl_(4), and fecl_(3) act as lewis acids because of the ability of their central atoms to accept lone pairs in their empty orbitals. Which one of following is the strongest acid;
The reaction of lewis acid/base forms a bond that is known as a coordinate covalent bond.
This has a cellular, trigonal planar geometry. Some of the common examples. alcl_(3), sncl_(4), and fecl_(3) act as lewis acids because of the ability of their central atoms to accept lone pairs in their empty orbitals. Drag the appropriate items to their respective bins. In lewis acid many acid do not have protons. The most lewis acidic component of these is bf_3, followed by c_2h_5oh and nh_3.
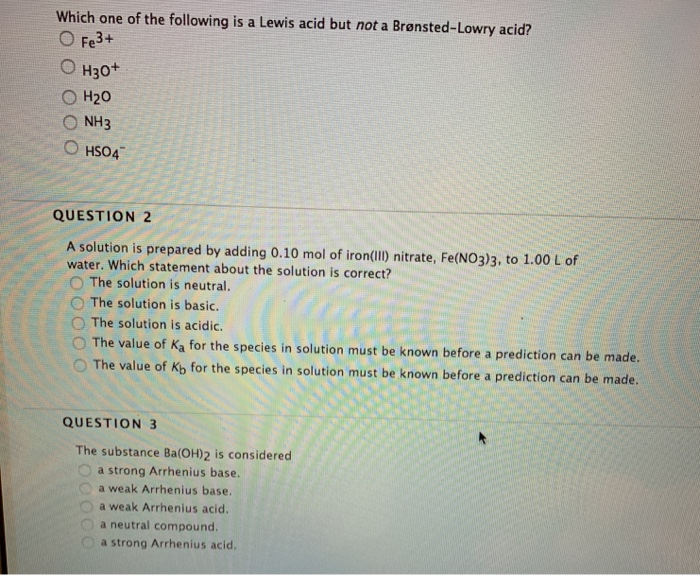 Source: chegg.com
Source: chegg.com
• it will react with a lewis base. Among the following compounds, the strongest acid is; It is electron deficient, so it can accept low pair of electron and behave as hewis acid.
 Source: chegg.com
Source: chegg.com
Which one of following is the strongest acid; The lewis acid strength of b b r 3. The most lewis acidic component of these is bf_3, followed by c_2h_5oh and nh_3.
 Source: youtube.com
Source: youtube.com
A lewis acid is a substance which accept electrons into an empty orbital. Increasing order of lewis acid strength; Question (16) which of the following is a lewis acid?
 Source: neetlab.com
Source: neetlab.com
The following equations illustrate the general application of the lewis concept. Bf_3 has an empty p orbital in which it can accept electrons. Which one of following is the strongest acid;
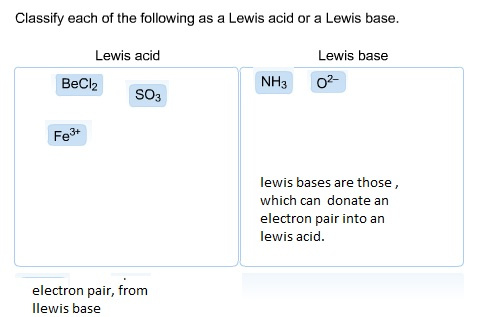 Source: ask.learncbse.in
Source: ask.learncbse.in
Is a radioactive boron and iodine compound with chemical formula bi3. Some of the common examples. • it has an incomplete octet.

We see that the reaction of a lewis acid and a lewis base, leads to the production of a coordinate covalent bond. The most lewis acidic component of these is bf_3, followed by c_2h_5oh and nh_3. This is a crystalline solid containing hydroiodic acid and boric acid, which reacts vigorously with water.
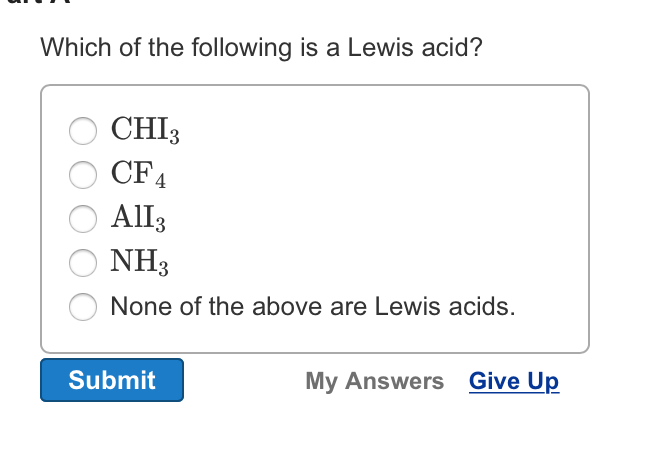 Source: chegg.com
Source: chegg.com
3.ammonia acts as a reducing agent. Which one of following is the strongest acid; The lewis acid strength of b b r 3.
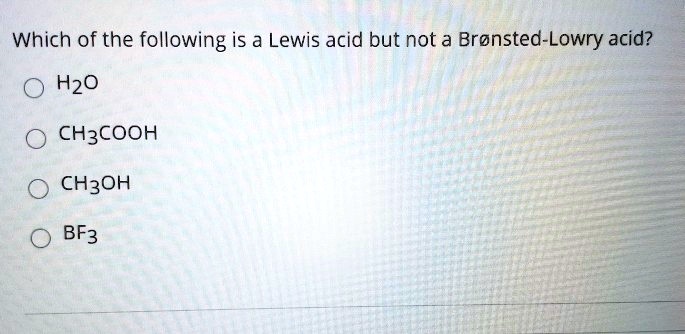 Source: numerade.com
Source: numerade.com
There are a number of reactions that involves lewis acids and bases. • it has an incomplete octet. The increasing order of acid strength of the following acid is:
 Source: doubtnut.com
Source: doubtnut.com
Which of the following is a lewis base? Some of the examples of lewis acids. The most lewis acidic component of these is bf_3, followed by c_2h_5oh and nh_3.
![Classify Each Of The Following As A Lewis Acid Or A Lewis Base. [{Image Src=�Acid_Base6618896991065085188.Jpg� Alt=�Acid Base� Caption=��}] | Study.com Classify Each Of The Following As A Lewis Acid Or A Lewis Base. [{Image Src=�Acid_Base6618896991065085188.Jpg� Alt=�Acid Base� Caption=��}] | Study.com](https://study.com/cimages/multimages/16/acid_base6618896991065085188.jpg) Source: study.com
Source: study.com
Becl2 is is a lewis acid. incl_(3) the order of decreasing lewis acid character is asked jan 25, 2021 in. Which of the following statements about lewis acids is true?
 Source: oneclass.com
Source: oneclass.com
Which of the following is a lewis acid (b) bf349 (c) nf3 no (d) oh no more than one of these choices. incl_(3) the order of decreasing lewis acid character is asked jan 25, 2021 in.
 Source: youtube.com
Source: youtube.com
(b) h2so4 + ch,co2na (d) ho+ ch,nh (e) two of these choices As a result, the boron atom is s p 2 hybridized, which leaves an empty 2 p z orbital on the boron atom. Which of the following are lewis acids and which are lewis bases?

B) lewis acids are proton acceptors. 13 a l = 2, 8, 3 and 17 c l = 2, 8, 7. A lewis base is a type of species that has the ability to donate a pair of electrons to the acceptor of the same category.
 Source: clutchprep.com
Source: clutchprep.com
Question (16) which of the following is a lewis acid? This means lewis acids are electrophilic in nature, meaning that they attract electrons. alcl_(3).6h_(2)o or [al(h_(2)o)_(6)]cl_(3) cannot act as a lewis acid because the central.
 Source: clutchprep.com
Source: clutchprep.com
Which one of following is the strongest acid; Thus, all cations are lewis acids since they are able to accept electrons. According to lewis concept of acids and bases, an acid is a substance which can accept a lone pair of electrons whereas a base is a substance which can donate a lone pair of electrons.
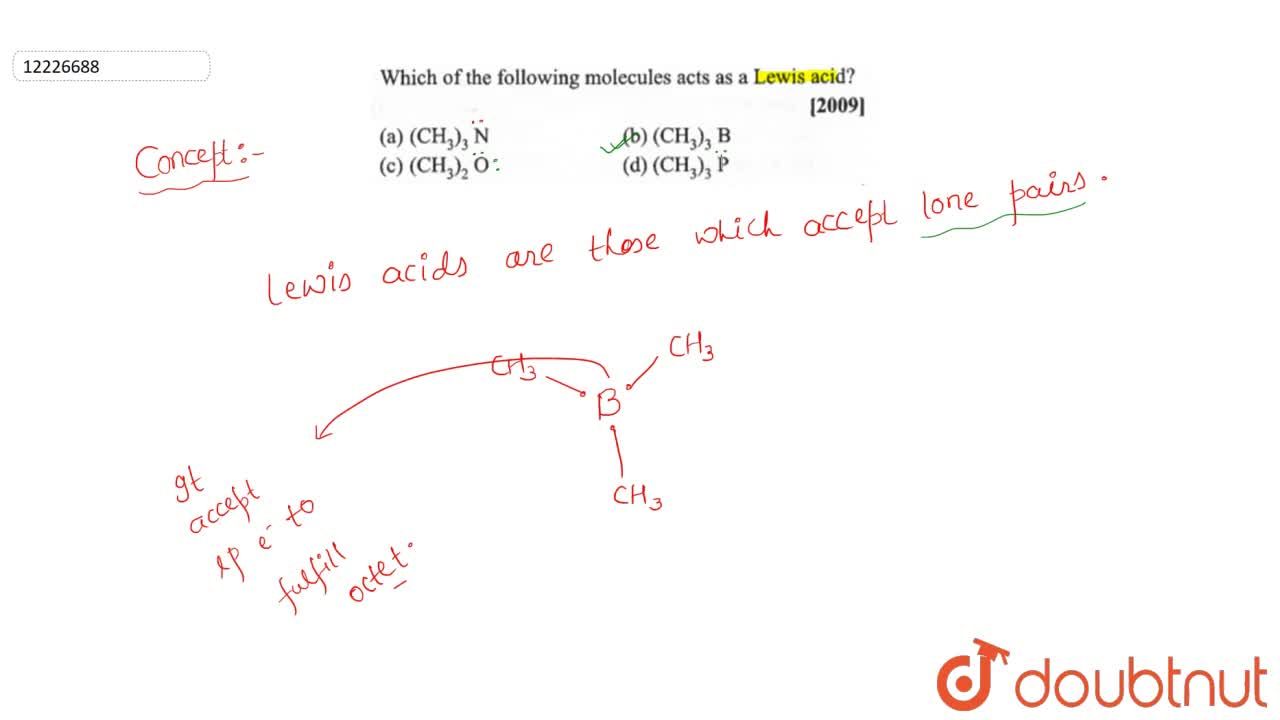 Source: doubtnut.com
Source: doubtnut.com
Which of the following is a lewis acid? In the reaction, s n c l 2 + 2 c l − → s n c l 4 , s n c l 2 is a lewis acid because s n c l 2 is a electron acceptor. Is a radioactive boron and iodine compound with chemical formula bi3.
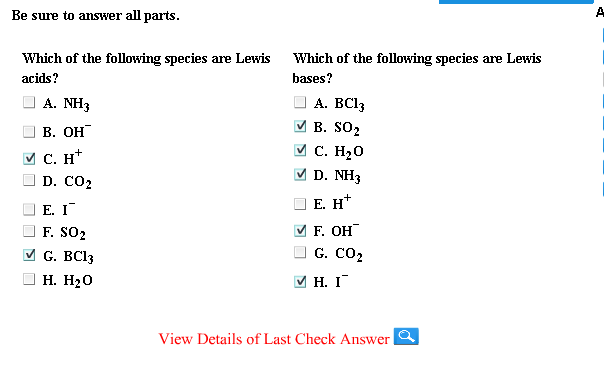 Source: chegg.com
Source: chegg.com
In lewis acid many acid do not have protons. B) lewis acids are proton acceptors. There are a number of reactions that involves lewis acids and bases.

Co 2 ( g ) + h 2 o( l ) h 2 co 3 ( aq ) in the course of this reaction, the water molecule acts as. incl_(3) the order of decreasing lewis acid character is asked jan 25, 2021 in. It is electron deficient, so it can accept low pair of electron and behave as hewis acid.
 Source: youtube.com
Source: youtube.com
Bf_3 has an empty p orbital in which it can accept electrons. As a result, the boron atom is s p 2 hybridized, which leaves an empty 2 p z orbital on the boron atom. Question (16) which of the following is a lewis acid?
 Source: toppr.com
Source: toppr.com
Thus, all cations are lewis acids since they are able to accept electrons. 3.ammonia acts as a reducing agent. Question (16) which of the following is a lewis acid?
Also Read :





