Is the following chemical equation balanced? What is chemical equation balancing?
Which Of The Following Is A Balanced Chemical Equation. Which of the following is a balanced chemical equation? Write the balanced chemical equation for the reaction shown. 2naoh + co₂ → na₂co₃ + h₂o Is the following chemical equation balanced?
 Writing And Balancing Chemical Equations | Introductory Chemistry – Lecture & Lab From courses.lumenlearning.com
Writing And Balancing Chemical Equations | Introductory Chemistry – Lecture & Lab From courses.lumenlearning.com
Related Post Writing And Balancing Chemical Equations | Introductory Chemistry – Lecture & Lab :
Why we should balance a chemical equation? A b a c l 2 ( a q ) + n a 2 s o 4 ( a q ) → b a s o 4 ( s ) + 2 n a c l ( a q ) The equation is balanced by changing the scalar numbers that precede each part of the equation. Balancing chemical equations involves the addition of stoichiometric coefficients to the reactants and products.
Magnesium is heated in air.
2na + 2h2o 2naoh + h2; 2na + 2h2o 2naoh + h2; Which of the following correctly represents a balanced chemical equation? Therefore, the balanced chemical equation is c 3 h 8 + 5o 2 → 3co 2 + 4h 2 o. Which of the following is a balanced chemical equation: H2so4 + ba (oh)2 → baso4 + h2o.

Of atoms and molecules in the equation whereas the balanced chemical equation shows. 2naoh + co₂ → na₂co₃ + h₂o H2 + o2 → h2o b.
 Source: brainly.com
Source: brainly.com
Therefore, the balanced chemical equation is c 3 h 8 + 5o 2 → 3co 2 + 4h 2 o. Let�s take a look at an equation representing. Alcl3 + li → al + licl :
 Source: youtube.com
Source: youtube.com
The equation in which the number of atoms of all the molecules is equal on both sides of the equation is known as a balanced chemical equation. After a visual inspection we see that all the atoms are completely balanced with equal numbers on both sides of reaction. The coefficients in a balanced chemical equation always can express the ratio of;
 Source: brainly.in
Source: brainly.in
A balanced chemical equation possesses an equal number of atoms at both sides of the equation. 2a + 2b → 2ab Write the balanced chemical equation for the reaction shown.
 Source: numerade.com
Source: numerade.com
Ch 4 + 2o 2 → co 2 + h c. The difference between these equations is that the skeleton equation doesnot show the no. Limiting reagent can be computed for a balanced equation by entering the number of moles or weight for all reagents.
 Source: brainly.in
Source: brainly.in
What is the balanced chemical equation for the reaction used to calculate δh∘f of caco3(s)? The equation is balanced by changing the scalar numbers that precede each part of the equation. Which of the following is a balanced chemical equation?
 Source: bartleby.com
Source: bartleby.com
Why we should balance a chemical equation? Mgf 2 + li 2 co 3 → mgco 3 + lif b. Fe(s) + h 2 o(g) $ fe 3 o 4 (s).
The reason for balancing equations in chemistry is to obtain the correct proportions of reagents and products for a given reaction.since chemical reactions do not change the atoms themselves, a balanced equation tells you how much product to expect for a given set of reagents. [cbse 2008] 2feso 4 (s) heat fe 2 o 3 (s) + so 2 (g) + so 3 (g) 33. The difference between these equations is that the skeleton equation doesnot show the no.
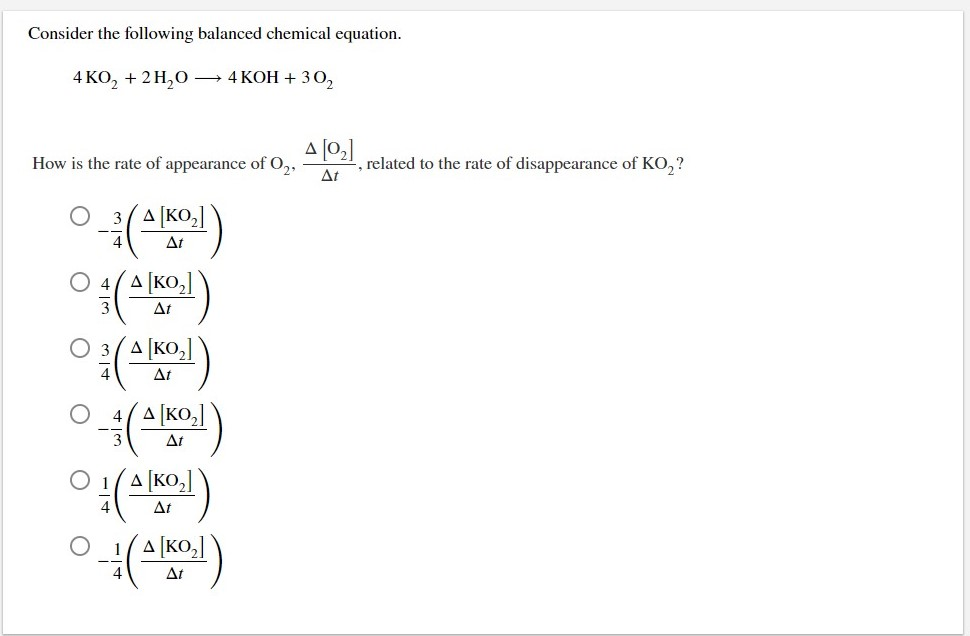 Source: chegg.com
Source: chegg.com
Which of the following is a balanced chemical equation: Therefore, the balanced chemical equation is c 3 h 8 + 5o 2 → 3co 2 + 4h 2 o. After a visual inspection we see that all the atoms are completely balanced with equal numbers on both sides of reaction.
 Source: brainly.com
Source: brainly.com
A balanced chemical equation possesses an equal number of atoms at both sides of the equation. Formula of a reactant or product cannot be altered, but chemical equation is balanced by multiplying the reactant or product with numbers like 2,3,4,5 and so on to balance the number of atoms in the chemical equation. The reason for balancing equations in chemistry is to obtain the correct proportions of reagents and products for a given reaction.since chemical reactions do not change the atoms themselves, a balanced equation tells you how much product to expect for a given set of reagents.
 Source: slideplayer.com
Source: slideplayer.com
Examples of complete chemical equations to. Nh3 + hcl → nh4cl: 2al + 6hcl → 3h 2 + alcl 3 d.
 Source: brainly.in
Source: brainly.in
What is the balanced chemical equation for the reaction used to calculate δh∘f of caco3(s)? This equation is balanced as it has one nitrogen atom, four hydrogen atoms and one chlorine atom at each side of the equation. 2 n a o h + h 2 s o 4 → n a 2 s o 4 + 2 h 2 o.
 Source: toppr.com
Source: toppr.com
Is the following chemical equation balanced? Nh3 + hcl → nh4cl: The equation in which the number of atoms of all the molecules is equal on both sides of the equation is known as a balanced chemical equation.
 Source: clutchprep.com
Source: clutchprep.com
Word equation is a balanced chemical equation [cbse 2008(c)] 2al(s) + 3cucl 2 (aq) $ 2alcl 3 (aq) + 3cu(s) 32. After a visual inspection we see that all the atoms are completely balanced with equal numbers on both sides of reaction.
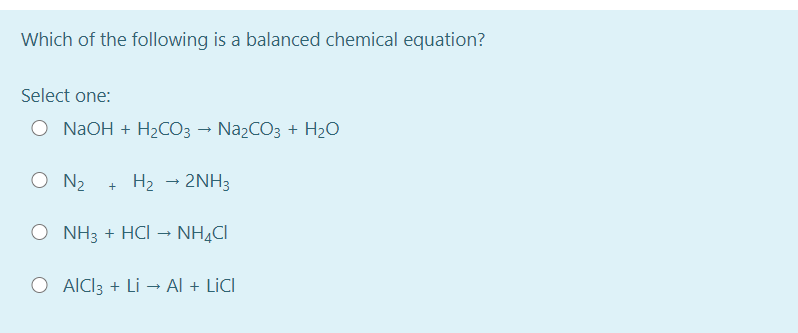 Source: bartleby.com
Source: bartleby.com
Is the following chemical equation balanced? What is the purpose of balancing chemical equations? Which of the following correctly represents a balanced chemical equation?
 Source: brainly.com
Source: brainly.com
Which of the following is a balanced chemical equation: Mgf 2 + li 2 co 3 → mgco 3 + lif b. Which of the following is a balanced chemical equation?
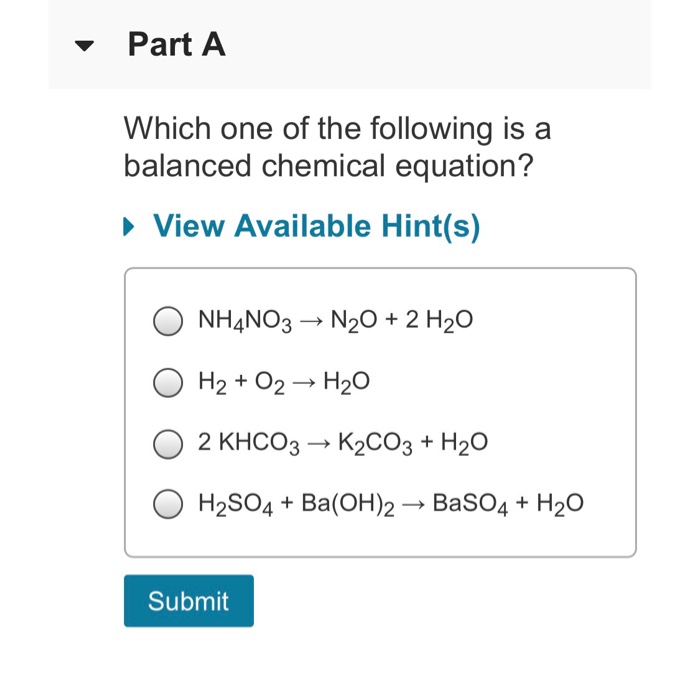
P 4 + 3o 2 → 2p 2 o 3 solution: Balance the given chemical equation: Feso 4 (s) heat fe 2 o 3 (s) + so (g) + so (g) ans :
 Source: youtube.com
Source: youtube.com
H2 + o2 → h2o b. What is chemical equation balancing? Which of the following is a balanced chemical equation:
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
The limiting reagent row will be highlighted in pink. After a visual inspection we see that all the atoms are completely balanced with equal numbers on both sides of reaction. 2al + 6hcl → 3h 2 + alcl 3 d.
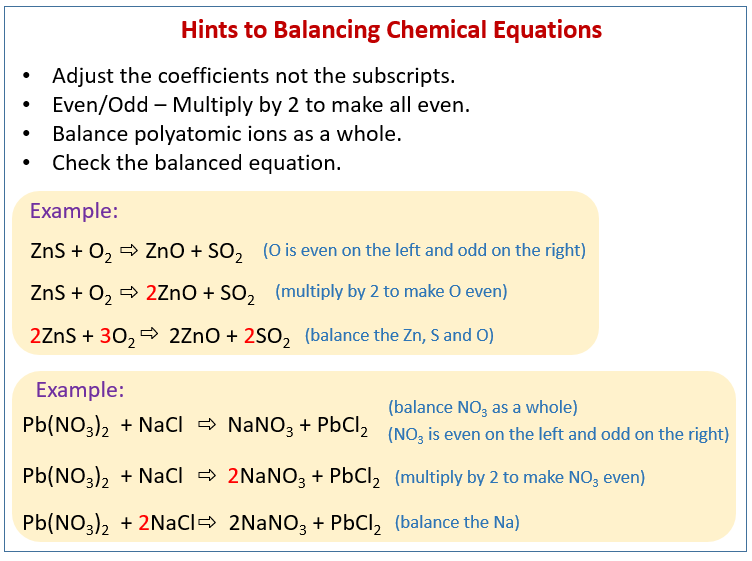 Source: onlinemathlearning.com
Source: onlinemathlearning.com
2naoh + co₂ → na₂co₃ + h₂o Limiting reagent can be computed for a balanced equation by entering the number of moles or weight for all reagents. Why we should balance a chemical equation?
Also Read :