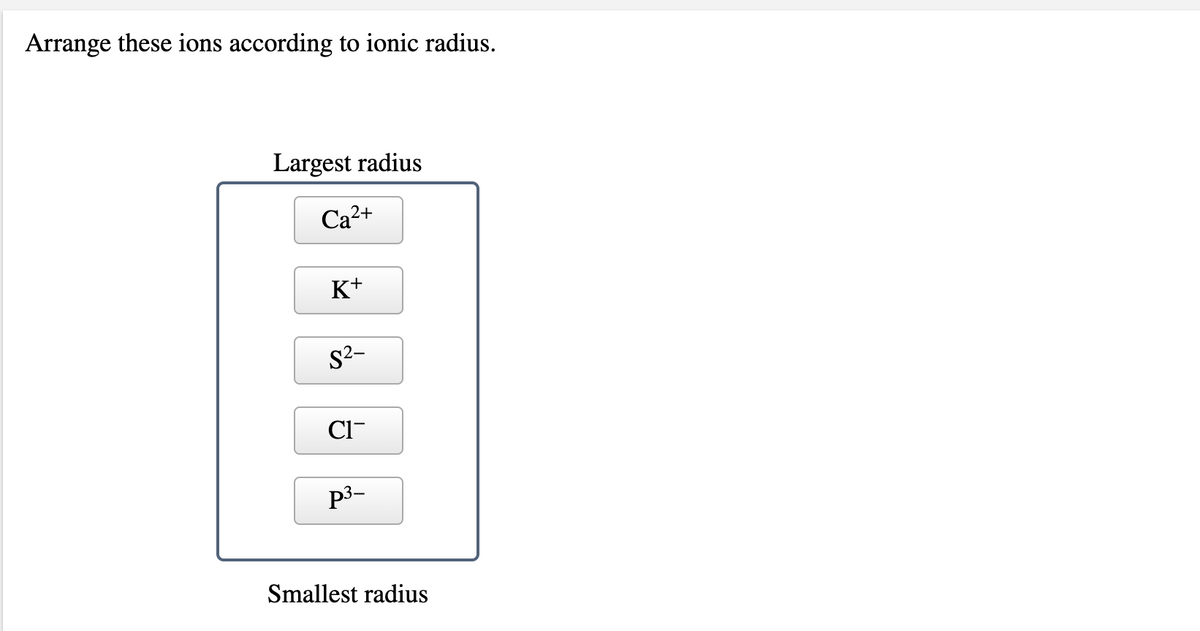
This energy level is expressed in the form of electron configurations. (a) each of the given species (ions) has the same number of electrons (10 electrons).
Which Of The Following Ions Has The Smallest Radius. Which ion in the isoelectronic series below has the smallest radius in a crystal? Therefore, the ion with the. Which one of the following ion has smallest radius? The more positive the charge of an ion is, the smaller the ion is.
 Periodic Trends Atomic Radii Elemental Properties And Patterns From slidetodoc.com
Periodic Trends Atomic Radii Elemental Properties And Patterns From slidetodoc.com
Related Post Periodic Trends Atomic Radii Elemental Properties And Patterns :
Hence, the ionic radius of mg2+ is smallest. The ionic radius of p t 2 + is maximum as it has two more shells of electrons than other ions. Heliumtherefore, rubidium has the largest atomic radius whereas helium has the smallest. The more positive the charge of an ion is, the smaller the ion is.
A) br b) as c) ca d) k.
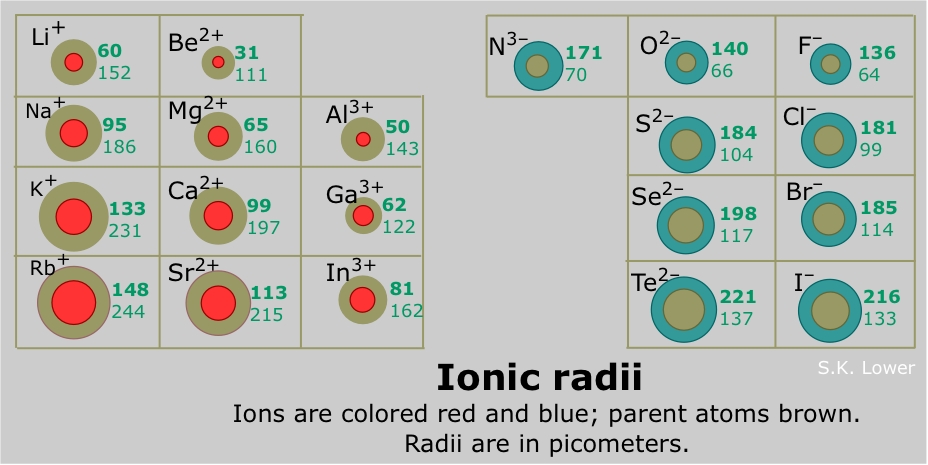
The more positive the charge of an ion is, the smaller the ion is. Size of mg is less than that of li because of the zeff of mg. O²⁻ ion has the smallest radius. Which atom has the lowest first ionization energy? Having same number of electrons (18) but their atomic numbers are. Ionic size varies inversely with nuclear charge.

This energy level is expressed in the form of electron configurations. A) argon b) neon c) helium d) krypton 6 using shorthand nation, the ground sae election conualin to 7. What elements have the smallest atomic radius?
 Source: bartleby.com
Source: bartleby.com
Further explanation in an atom there are levels of energy in the shell and sub shell. View solution > among the following which is smallest anion according to ionic radius? Which of the following atoms has the smallest radius?
 Source: chem.libretexts.org
Source: chem.libretexts.org
Mg2+ = 12 protons, 10 electrons. O²⁻ ion has the smallest radius. Ionic size varies inversely with nuclear charge.
 Source: socratic.org
Source: socratic.org
Heliumhelium has the smallest atomic radius. Which of the following elements has the smallest atomic radius? Which one of the following ion has smallest radius?
View solution > which of the following is the smallest ion? In the third transition series, the ionic radius of z n 2 + is maximum due to interelectronic repulsion in 3d orbital because of completely filled d orbital. Which ion has the largest radius?
 Source: actingcolleges.org
Source: actingcolleges.org
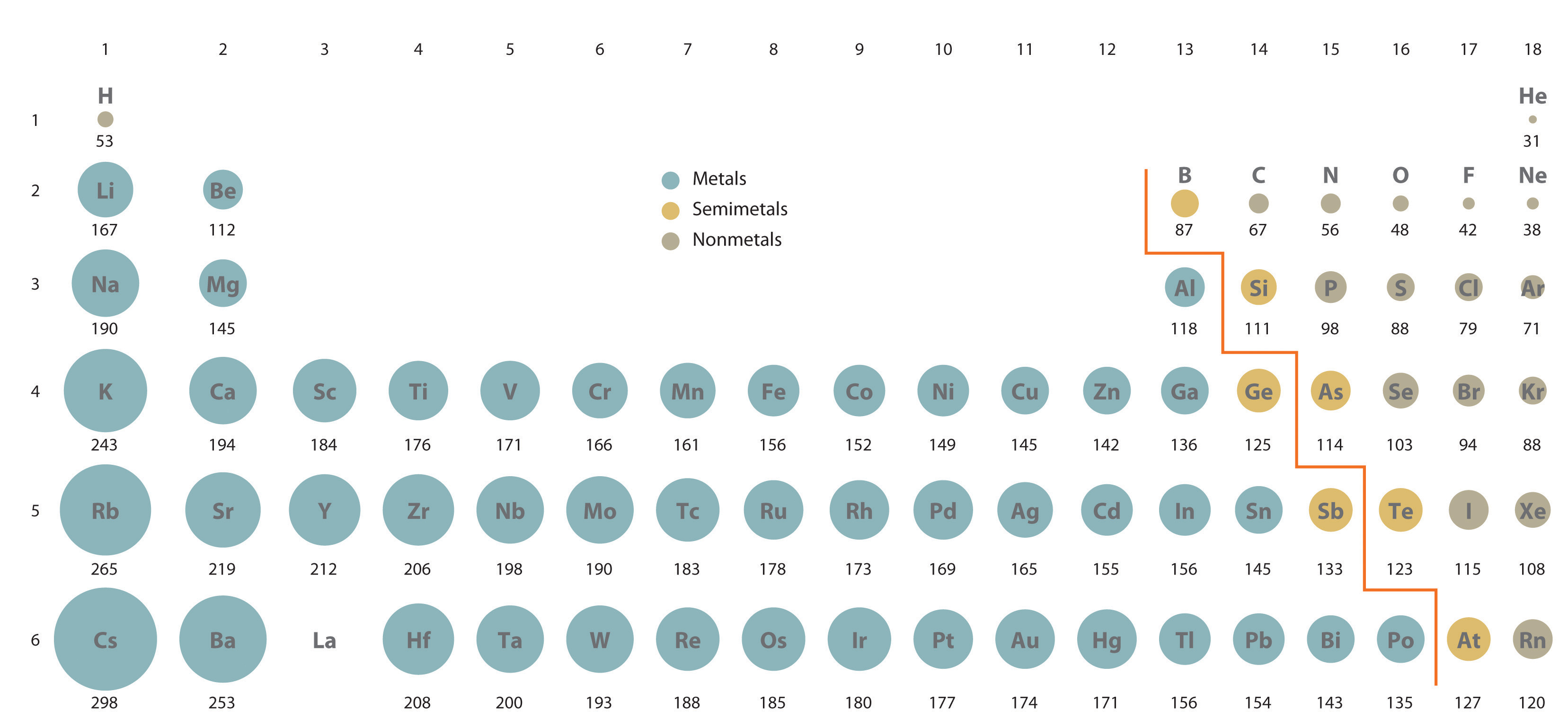
The ionic radius of ni2+ is minimum. This is due to trends in the periodic table, and the effective nuclear charge that holds the valence electrons close to the nucleus. Which of the following atoms has the largest radius?
 Source: doubtnut.com
Source: doubtnut.com
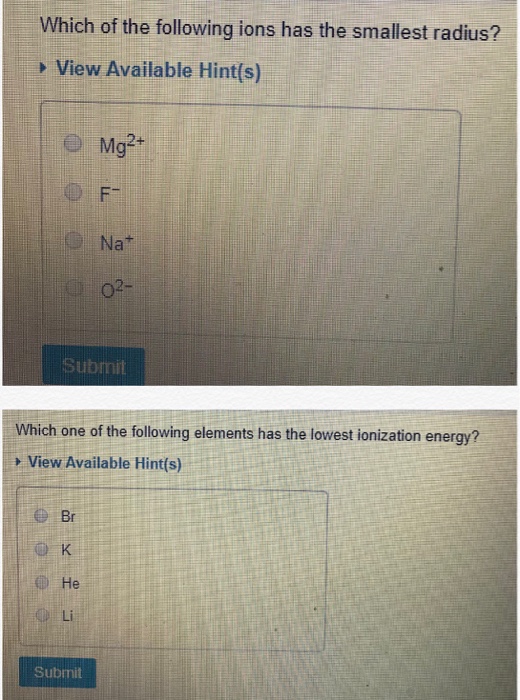
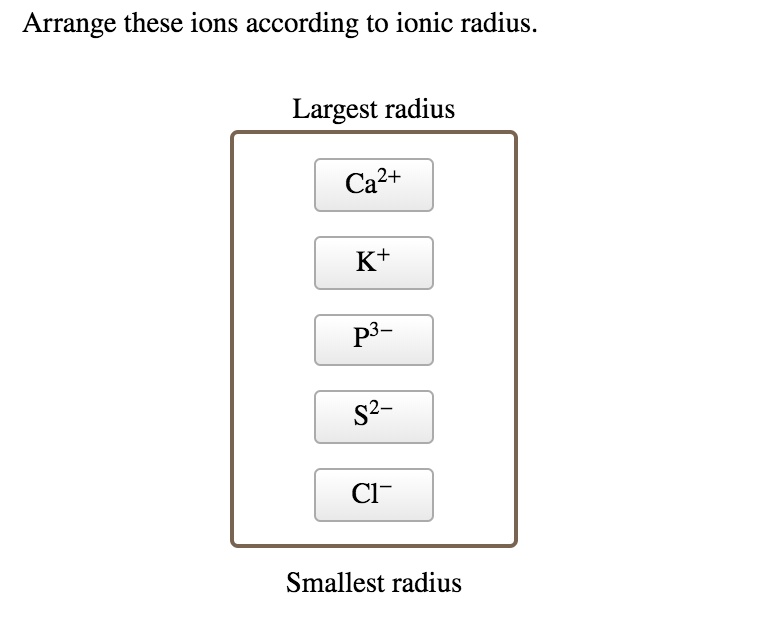
Which of the following ions has the smallest radius? Cations have smaller ionic radii than their neutral atoms. Which of the following atoms has the smallest radius?
 Source: numerade.com
Source: numerade.com
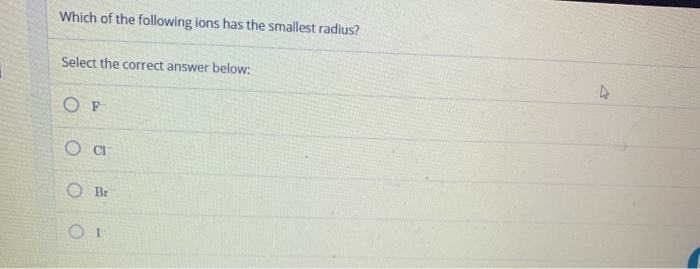
If we compare according to the increase and decrease along with the period and group then zinc should have a smaller radius than a nickel. Which of the following atoms has the largest radius? A) rb b) si c) s d) o.
 Source: chegg.com
Source: chegg.com
So, the correct answer is option c. Which atom has the lowest first ionization energy? Which of the following atoms has the smallest radius?
 Source: toppr.com
Source: toppr.com
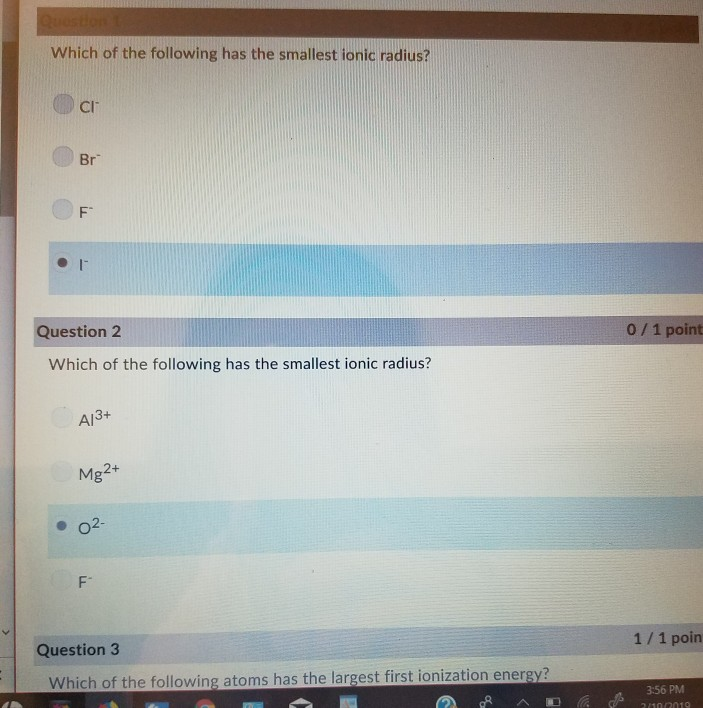
Which ion in the isoelectronic series below has the smallest radius in a crystal? A) br b) as c) ca d) k. View solution > which of the following ions has the second smallest radius?
 Source: chegg.com
Source: chegg.com
View solution > which of the following ions has the second smallest radius? As the protons are added, electrons are attracted with a greater strength towards the core, making the radius of mg smaller. Cations have smaller ionic radii than their neutral atoms.
 Source: slideplayer.com
Source: slideplayer.com
Which of the following ions has the smallest radius? The ionic radius of ni2+ is minimum. K and i which of the following 3rd period elements has the smallest atomic radium?
 Source: oneclass.com
Source: oneclass.com
Which of the following ions has the smallest radius? Which one of the following ion has smallest radius? Which has the smallest size?
 Source: chem.libretexts.org
Source: chem.libretexts.org
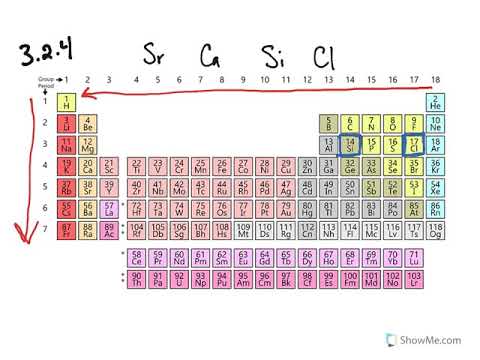
What elements have the smallest atomic radius? A) argon b) neon c) helium d) krypton 6 using shorthand nation, the ground sae election conualin to 7. View solution > which of the following is the smallest ion?
 Source: clutchprep.com
Source: clutchprep.com
So therefore, elements with a positive charge, will have a smaller ionic radius. Having same number of electrons (18) but their atomic numbers are. Therefore, the ion with the.
 Source: transtutors.com
Source: transtutors.com
Atomic radius decreases as you move across a period from left to right and decreases as you move up a group from bottom to top. If we compare according to the increase and decrease along with the period and group then zinc should have a smaller radius than a nickel. Therefore, the ion with the.
 Source: youtube.com
Source: youtube.com
(a) each of the given species (ions) has the same number of electrons (10 electrons). This means ca2+ will have the smallest radius since it has the most positive charge. Which ion has the largest radius?
![Solved] Which Of The Following Ions Has The Smallest Radius? | Course Hero](https://www.coursehero.com/qa/attachment/10691057/ “Solved] Which Of The Following Ions Has The Smallest Radius? | Course Hero”) Source: coursehero.com
(a) each of the given species (ions) has the same number of electrons (10 electrons). Which ion in the isoelectronic series below has the smallest radius in a crystal? Because of this, the ionic radius will just depend on the charge of the ion:
 Source: slidetodoc.com
Source: slidetodoc.com
Radius is smaller since it is missing an electron. So therefore, the above ions all have 10 electrons in their shells and magnesium with atomic number 12 and having lost 2 electrons to become positively charge has the smallest radius compared to others. View solution > which of the following ions has the second smallest radius?
 Source: youtube.com
Source: youtube.com
Which one of the following ion has smallest radius? In the third transition series, the ionic radius of z n 2 + is maximum due to interelectronic repulsion in 3d orbital because of completely filled d orbital. Which of the following anion has the smallest radius?
Also Read :





