Tetrahydrofuran is a heterocyclic compound. What is the most likely structure of this compound?
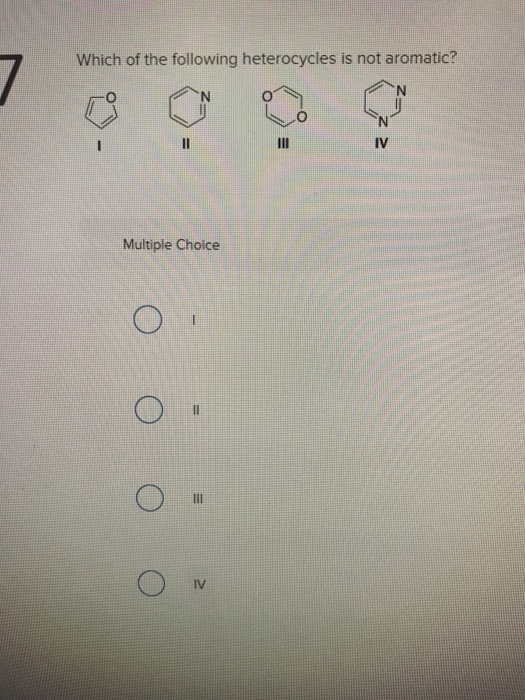
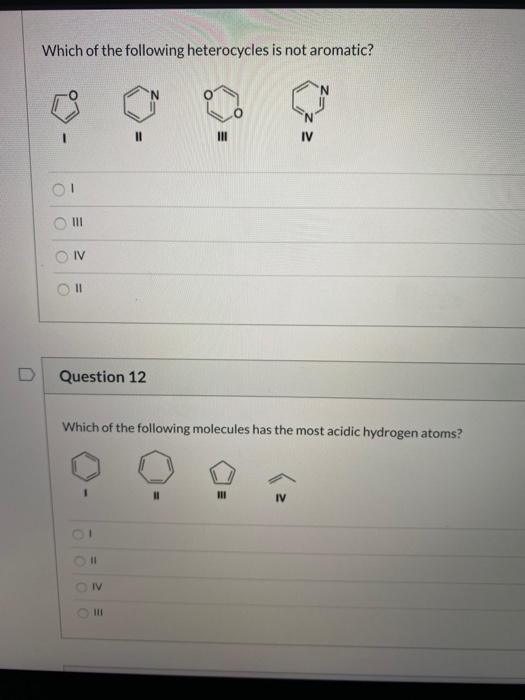
Which Of The Following Heterocycles Is Not Aromatic. So we might consider these three aromatic hetero cycles as examples. Thiophene is a heterocyclic compound with the formula c 4 h 4 s. A) i b)ii c)iii d)iv. 19)which of the following molecules has the most acidic hydrogen atoms?
 Which Of The Following Heterocycles Is Not Aromatic? | Study.com From study.com
Which Of The Following Heterocycles Is Not Aromatic? | Study.com From study.com
Related Post Which Of The Following Heterocycles Is Not Aromatic? | Study.com :
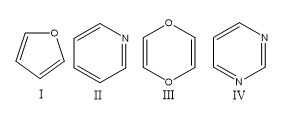
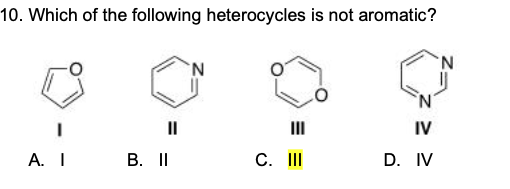
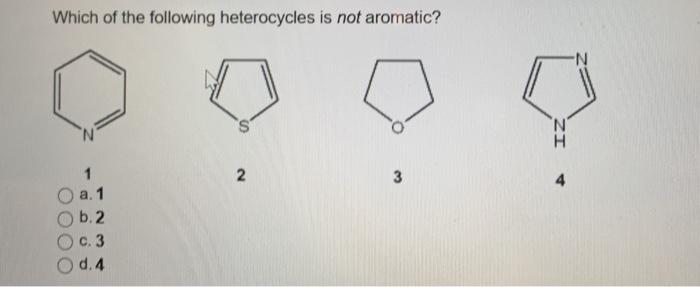
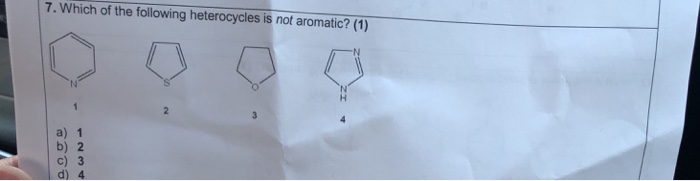
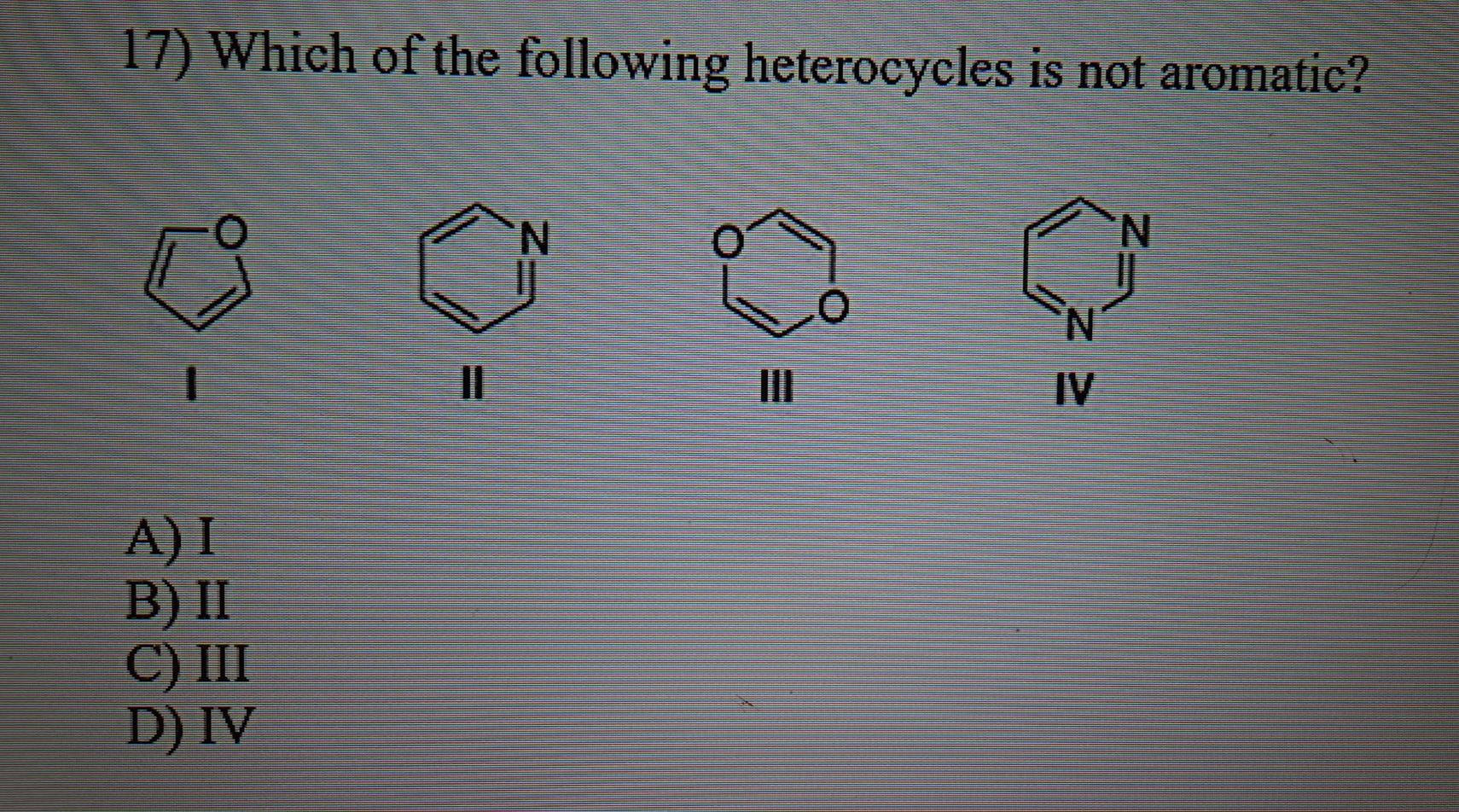
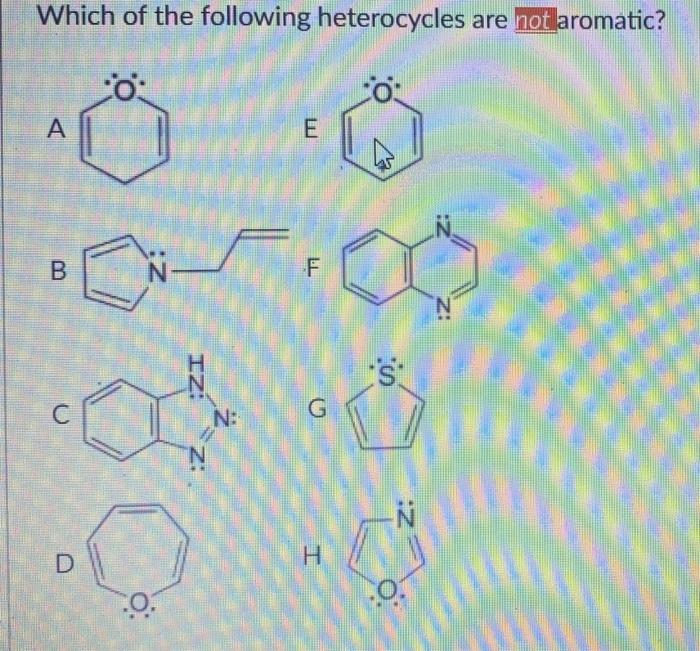
If assertion is true but reason is false. According to huckel�s rule, a cyclic, planar molecule is aromatic, when it has a conjugated system of [\left( {4n + 2} \right)\pi ] electrons. 17)which of the following heterocycles is not aromatic? It is aromatic as indicated by its extensive substitution reactions.
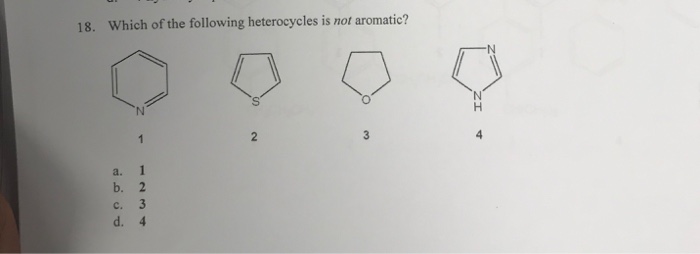
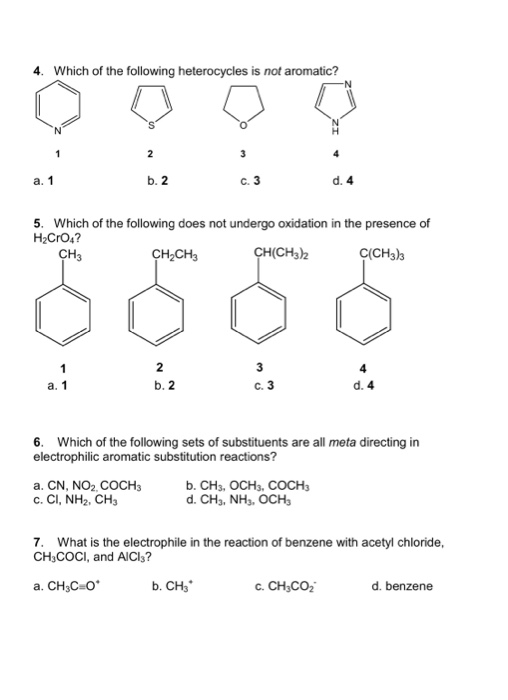
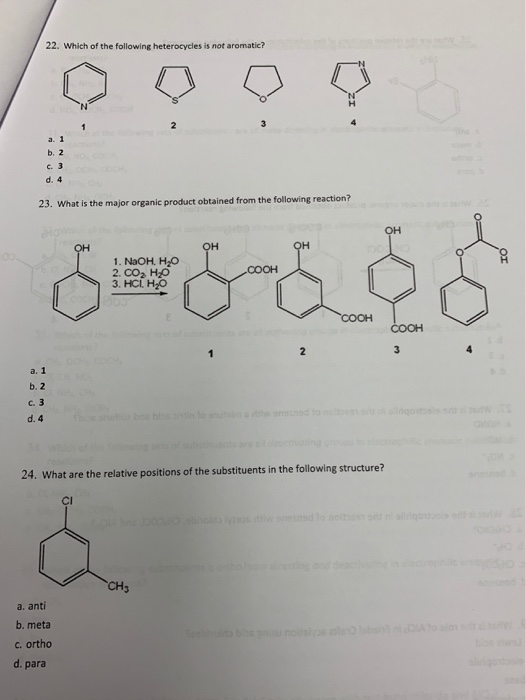
Which of the following heterocycles are not aromatic?
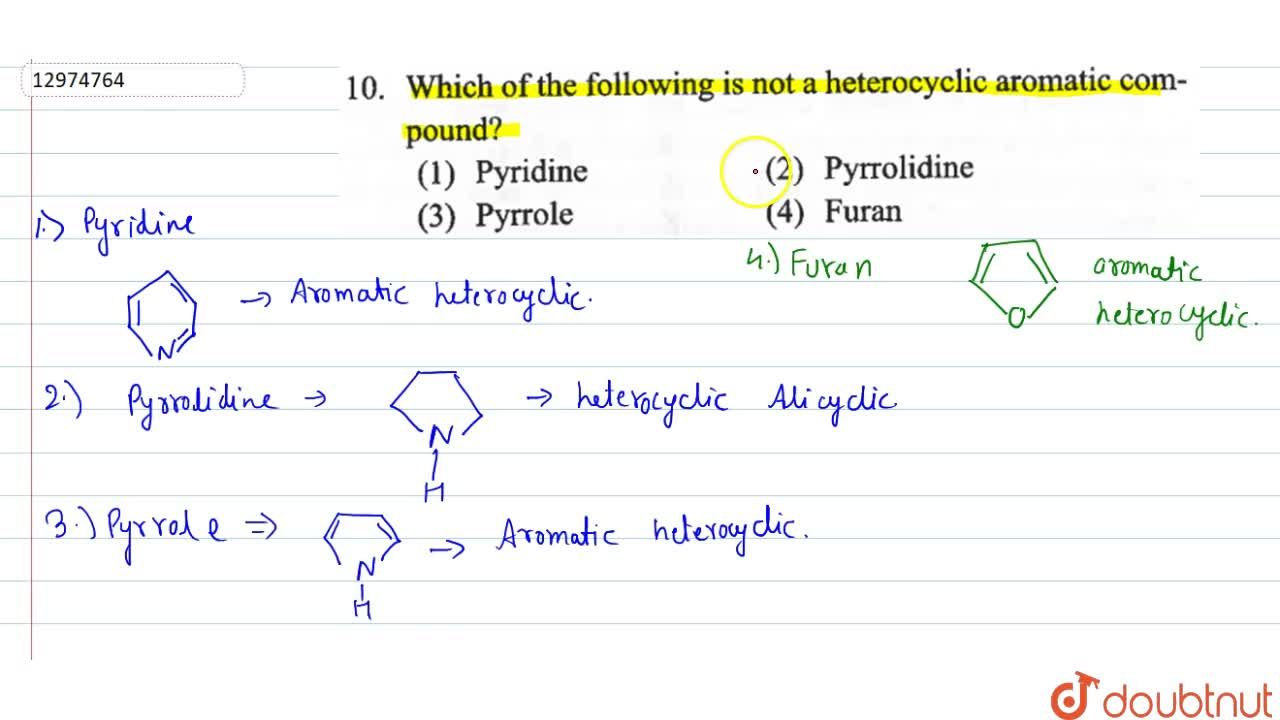
If both assertion and reason are true and reason is the correct explanation of assertion. Nitrogen, oxygen, sulphur are normal hetero atoms. C( (m 3 ) 3 Which of the following heterocycles is not aromatic? Which of the following is not a heterocyclic aromatic compound? Pyridine is cyclic, conjugated, and has three pi bonds.
 Source: bartleby.com
Source: bartleby.com
Which of the following is not a heterocyclic aromatic compound? Ii and iv only c. I and ii only b.
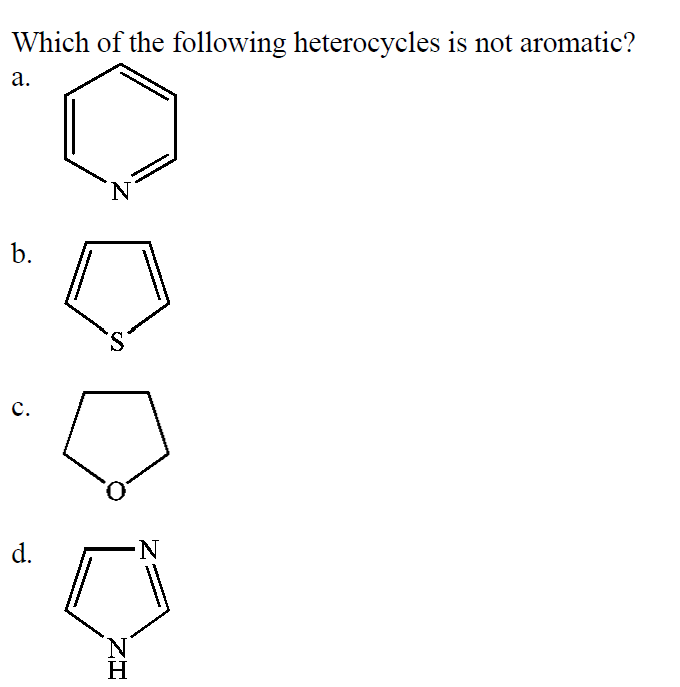
Which of the following heterocycles is not aromatic? Which of the following heterocycles is not aromatic? Which stucture is not aromatic?
If both assertion and reason are false. 17)which of the following heterocycles is not aromatic? Thiophene is a heterocyclic aromatic compound.
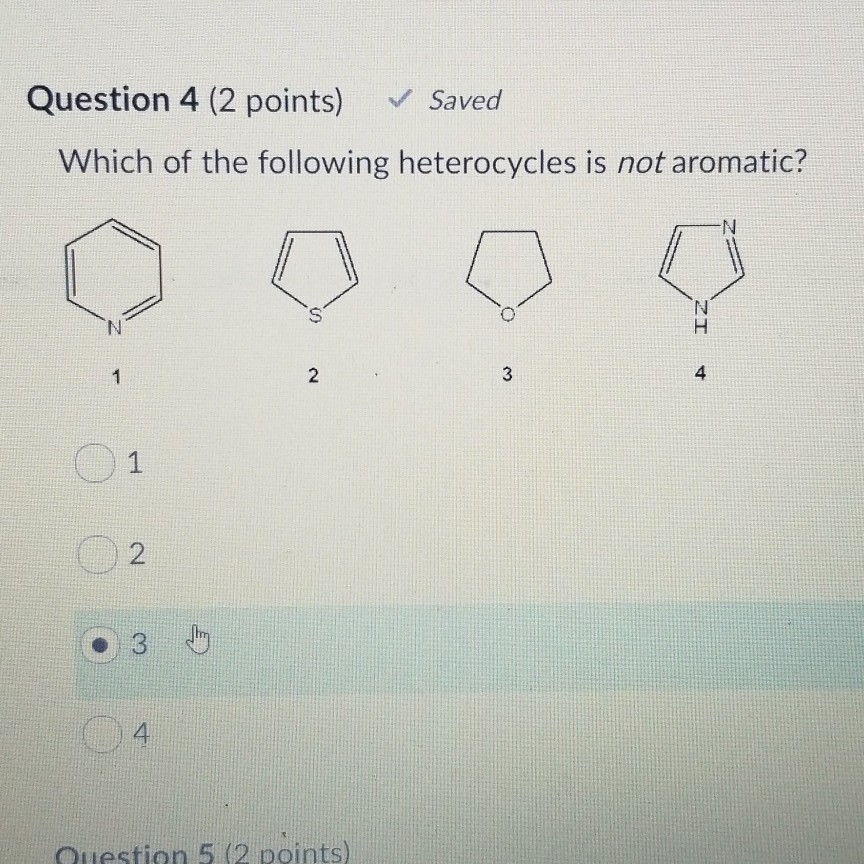 Source: chegg.com
Source: chegg.com
Then the probability that it was drawn from the bag containing the most black ball is. Which of the following heterocycles are not aromatic? In the option a ) cycloheptatrienyl anion has 8 pi electrons.
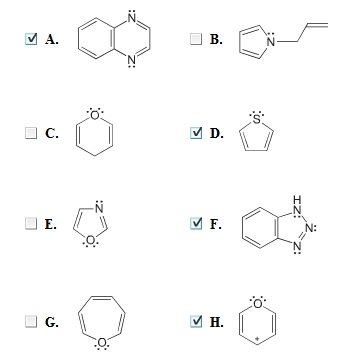 Source: clutchprep.com
Source: clutchprep.com
Hence, the correct option is option a ). Ii and iv only c. The number of the localized electrons has to be even but not a multiple of four.
 Source: youtube.com
Source: youtube.com
So four n plus two pi electrons, um, is gonna be aromatic. But it is not an aromatic compound. So in pure reading it looks like we might have eight electrons which of course break, would break.
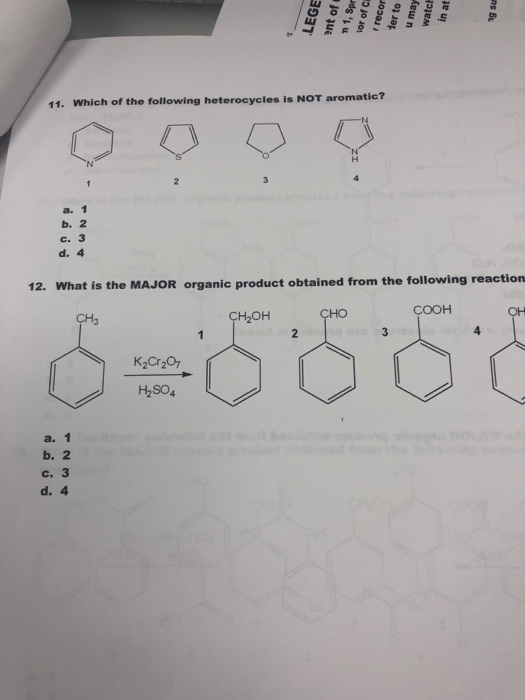 Source: chegg.com
Source: chegg.com
There are 3 bags which are known to contain 2 white and 3 black, 4 white and 1 black, and 3 white and 7 black ball, respectively. Thiophene is a heterocyclic aromatic compound. Problem number 33 from the smith organic chemistry textbook on in this problem were given eight header cycles and were asked which of the following head of hetero cycles are aromatic.
Naphthalene contains carbon and hydrogen atoms. According to huckel’s rule an aromatic compounds must be cyclic in nature with planar geometry due to conjugate double bonds and must have (4n+2)π electrons. I, ii and iv only d.
 Source: chegg.com
Source: chegg.com
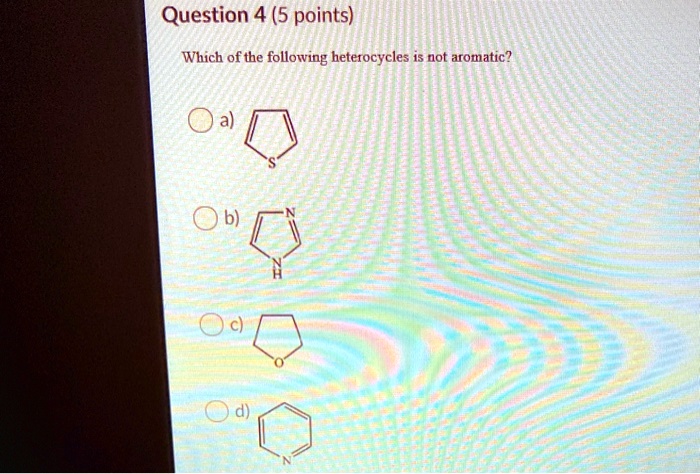
Which of the following compound is not aromatic? Which of the following compound is not aromatic? Which of the following is not a heterocyclic aromatic compound?
Thus the number of pi electrons in cycloheptatrienyl anion is not equal to 2,6,10, 14 hence, cycloheptatrienyl anion is not an aromatic compound. The number of the localized electrons has to be even but not a multiple of four. Tetrahydrofuran is a heterocyclic compound.
 Source: chegg.com
Source: chegg.com
If both assertion and reason are true and reason is the correct explanation of assertion. What is the correct assignment of the names of the following aromatic heterocycles? A ball is drawn at random from one of the bags and found to the black ball.
 Source: chegg.com
Source: chegg.com
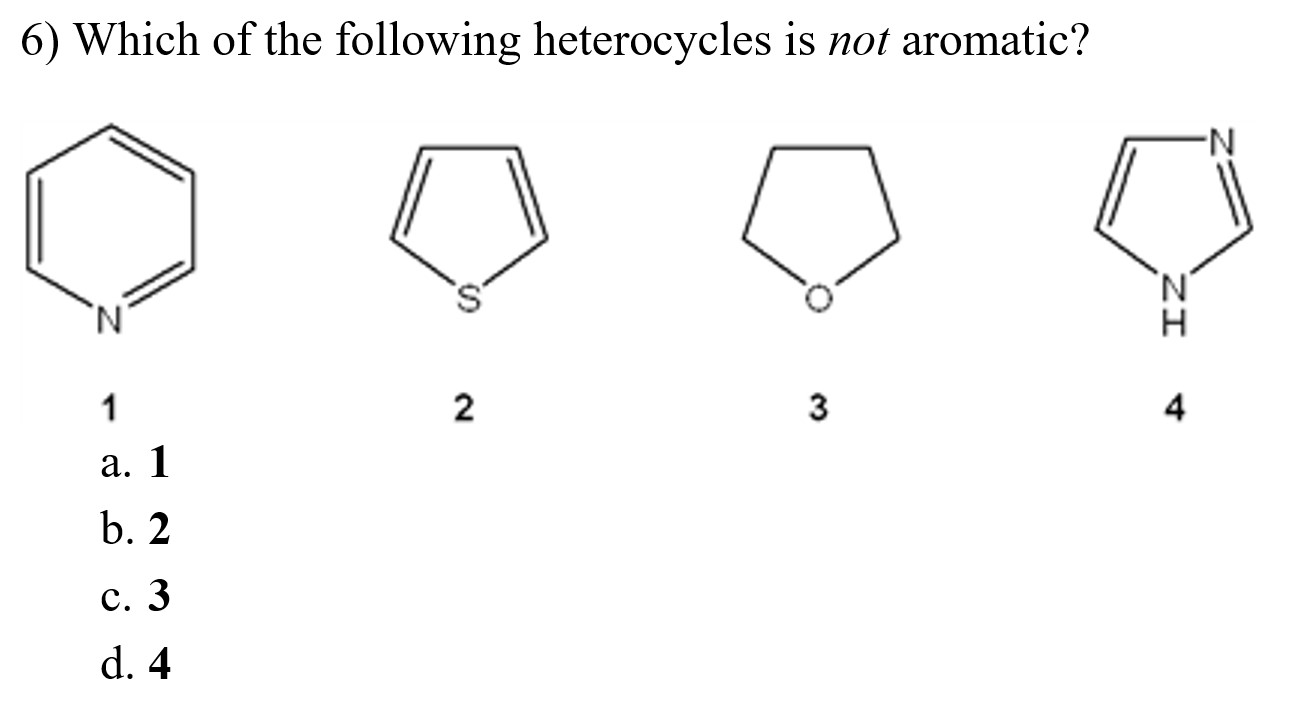
17)which of the following heterocycles is not aromatic? (a) pyridine (b) pyrrole (c) furan (d) piperidine A ball is drawn at random from one of the bags and found to the black ball.
What is the most likely structure of this compound? So, it is a heterocyclic aromatic compound. C( (m 3 ) 3
![Solved] Question 8 Which Of The Following Heterocycles Is Not Aromatic? I. 2 4 Oa. 1 Ob. 2 Oc.3 Od. 4 Question 9 Which Of The Following Ions Is Arom… | Course Hero](https://www.coursehero.com/qa/attachment/18677769/ “Solved] Question 8 Which Of The Following Heterocycles Is Not Aromatic? I. 2 4 Oa. 1 Ob. 2 Oc.3 Od. 4 Question 9 Which Of The Following Ions Is Arom… | Course Hero”) Source: coursehero.com
Which of the following heterocycles is not aromatic? Ii and iv only c. Pyridine is cyclic, conjugated, and has three pi bonds.
 Source: chegg.com
Source: chegg.com
Which of the following is not a heterocyclic aromatic compound? So in pure reading it looks like we might have eight electrons which of course break, would break. What is the correct assignment of the names of the following aromatic heterocycles?
 Source: numerade.com
Source: numerade.com
Which of the following is not a heterocyclic aromatic compound? Furan is heterocyclic aromatic species as it is planar, cyclic, conjugated. I, ii and iv only d.
 Source: chegg.com
Source: chegg.com
Furan is heterocyclic aromatic species as it is planar, cyclic, conjugated. In the option a ) cycloheptatrienyl anion has 8 pi electrons. So in pure reading it looks like we might have eight electrons which of course break, would break.
 Source: doubtnut.com
Source: doubtnut.com
Ii and iv only c. So in other words, it can be expressed by four n plus two. So four n plus two pi electrons, um, is gonna be aromatic.
 Source: study.com
Source: study.com
The number of the localized electrons has to be even but not a multiple of four. A ball is drawn at random from one of the bags and found to the black ball. Which of the following compound is not aromatic?
 Source: chegg.com
Source: chegg.com
Correct option is c) benzene contains carbon and hydrogen atoms. A ball is drawn at random from one of the bags and found to the black ball. Give two examples for each of the following type of organic compounds.
Also Read :





