Br2, f2, i2, cl2, answer higher boiling points will correspond to stronger intermolecular forces. Which has the lowest boiling point at 1 atmospheric pressure?
Which Of The Following Has The Lowest Boiling Point. Part b and c are done the same way. N2 br2 h2 cl2 o2 a)o2 b)br2 c)n2 d)h2 e)cl2 6) 7)in which of the following molecules is hydrogen bonding likely to be the most significant .fo 9) which of the following alkynes has the lowest boiling point? Due to which the attractive forces between the molecules (van der waals forces) decreases.
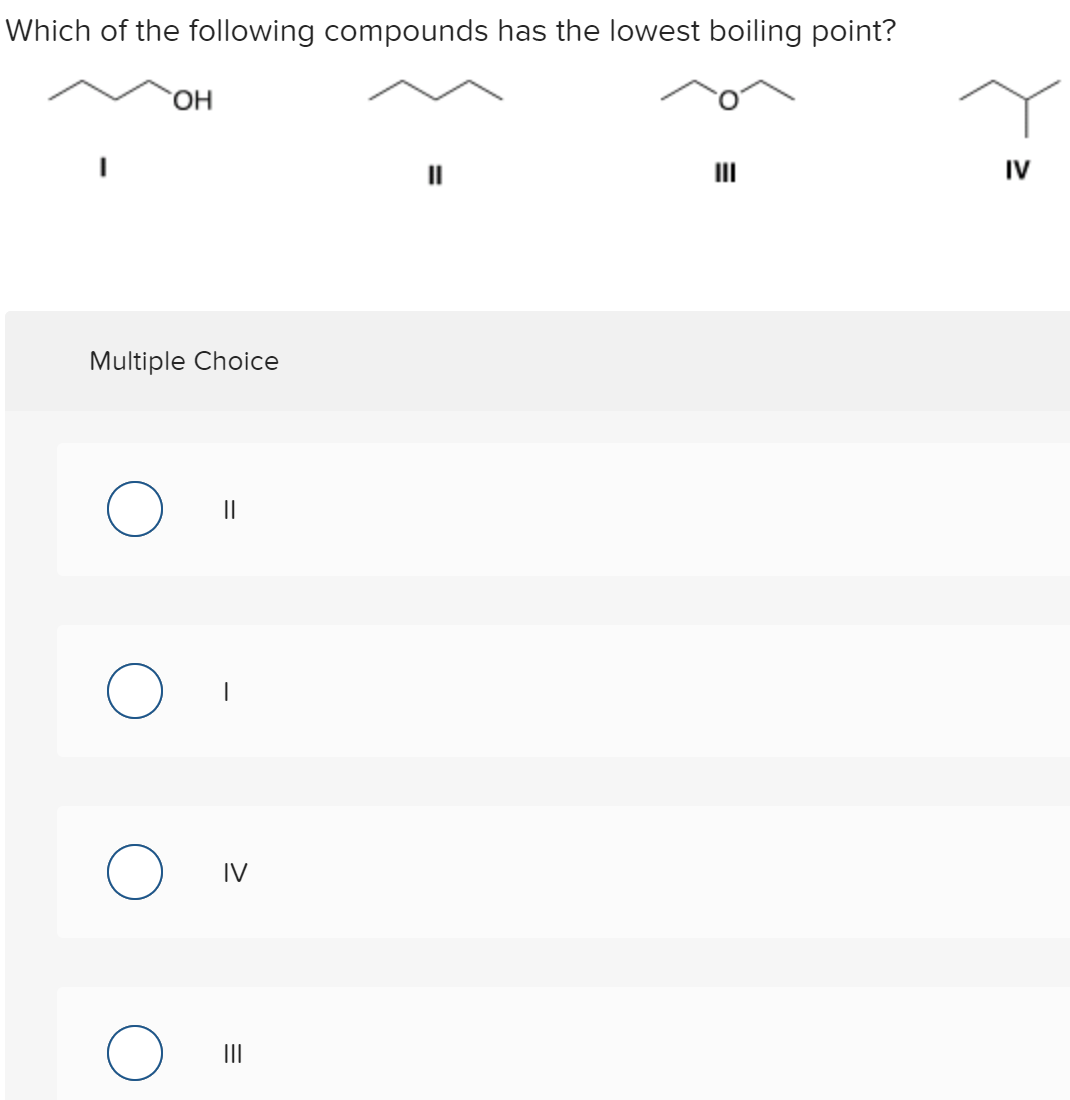 Answered: Which Of The Following Compounds Has… | Bartleby From bartleby.com
Answered: Which Of The Following Compounds Has… | Bartleby From bartleby.com
Related Post Answered: Which Of The Following Compounds Has… | Bartleby :
Closed nov 11, 2021 by swamijain. Due to least surface area, neopentane has the lowest b.p. 5)of the following substances, _____ has the highest boiling point. H2s < h2se < h2te < h2o this is the correct boiling point order.
The midweight alkanes are liquids;
N2 br2 h2 cl2 o2 a)o2 b)br2 c)n2 d)h2 e)cl2 6) 7)in which of the following molecules is hydrogen bonding likely to be the most significant The tabular chart on the right is arranged by. Which one of the following correctly ranks the compounds in order of lowest enthalpy of vaporization to highest enthalpy of vaporization based only in intermolecular forces? The boiling point of a liquid at 1 atm is also known as the normal boiling point. Dimethyl ether, ch_3och_3, is a polar molecule. The chemical element with the lowest boiling point is helium and the element with the highest boiling point is tungsten.
 Source: seniorcare2share.com
Source: seniorcare2share.com
Which one of the following correctly ranks the compounds in order of lowest enthalpy of vaporization to highest enthalpy of vaporization based only in intermolecular forces? Nh3 will have the highest boiling point because nh3 molecules can form hydrogen bonds with each other. Heliumthe chemical element with the lowest boiling point is helium and the element with the highest boiling point is tungsten.

It has been found that helium has the lowest normal boiling point (−268.9 °c) because it has very weak intermolecular attractions. Methanol has strong hydrogen bonds. Heliumthe chemical element with the lowest boiling point is helium and the element with the highest boiling point is tungsten.
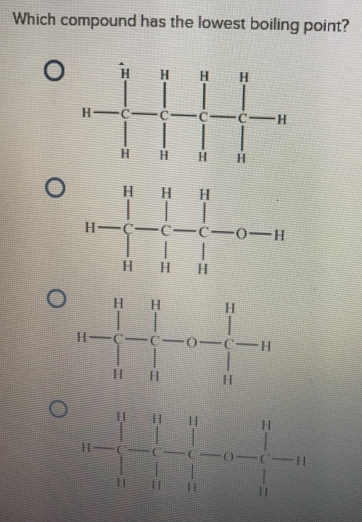 Source: clutchprep.com
Source: clutchprep.com
I for glucose is 1. I for bacl2 is 3. Nh3 will have the highest boiling point because nh3 molecules can form hydrogen bonds with each other.
 Source: chegg.com
Source: chegg.com
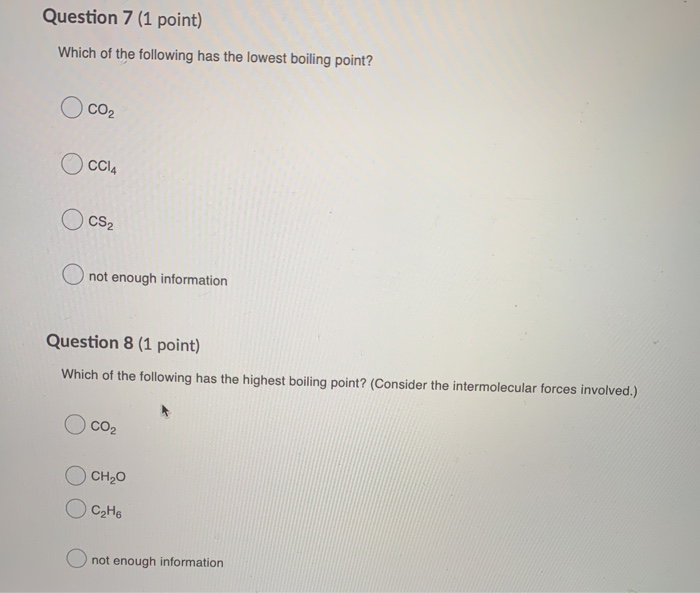
Which has lowest boiling point? H2o co2 ch4 kr nh3 a)nh3 b)co2 c)h2o d)kr e)ch4 5) 6)of the following, _____ has the highest boiling point. But due to high symmetry it has the highest m.p.
 Source: toppr.com
Source: toppr.com
So, in order of decreasing boiling point: If their policy has a deductible of $1,000, what is their. The chemical element with the lowest boiling point is helium and the element with the highest boiling point is tungsten.
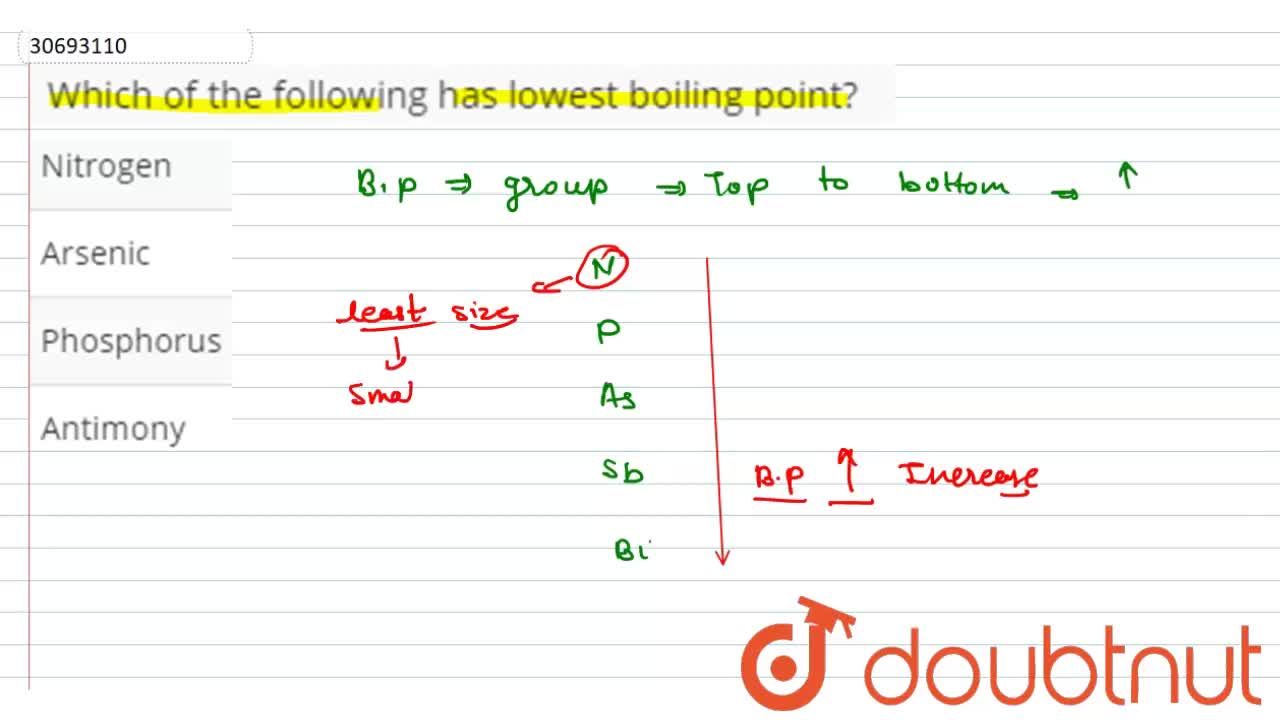 Source: doubtnut.com
Source: doubtnut.com
It has been found that helium has the lowest normal boiling point (−268.9 °c) because it has very weak intermolecular attractions. The lowest boiling point will be the lowest im which is sucrose. So the highest boiling point will be the one that has the highest im which is na2so4.
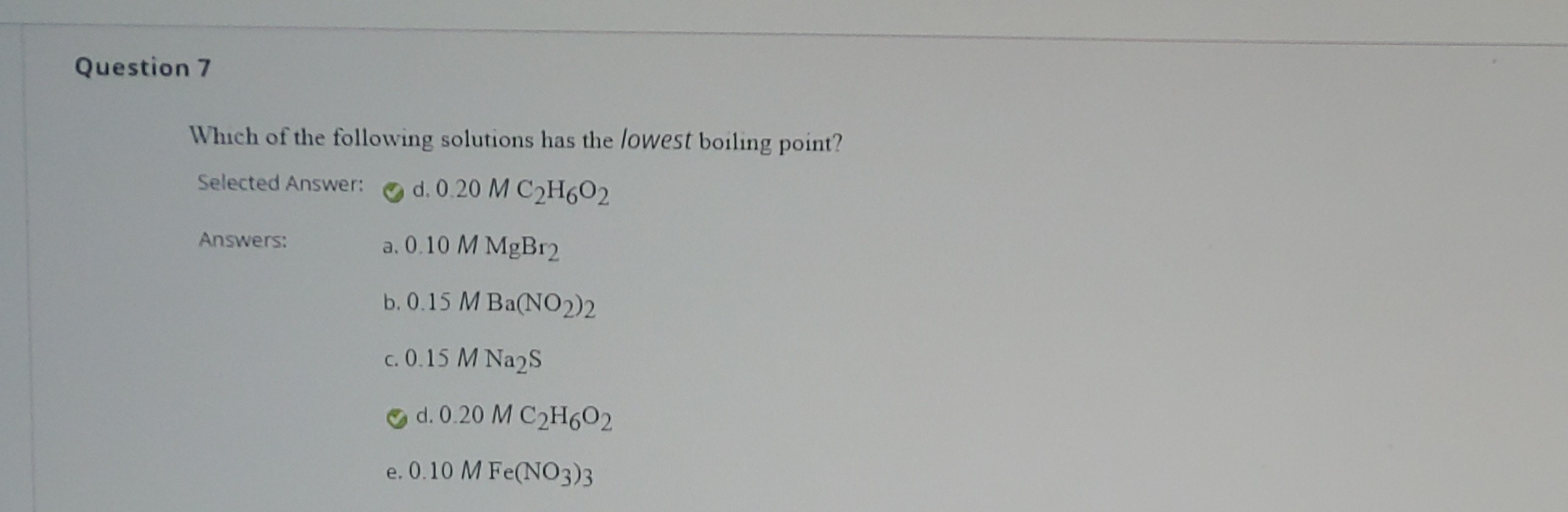 Source: clutchprep.com
Source: clutchprep.com
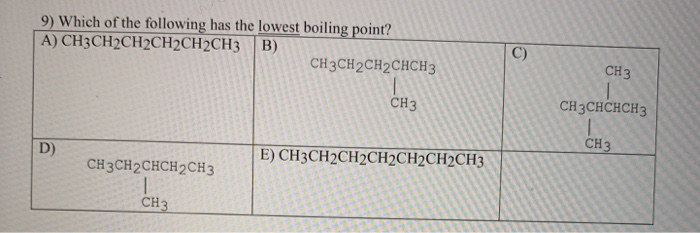
- which of the following alkynes has the lowest boiling point? None of these have hydrogen bonding. Among the following select the alkane that is expected to have lowest boiling point.
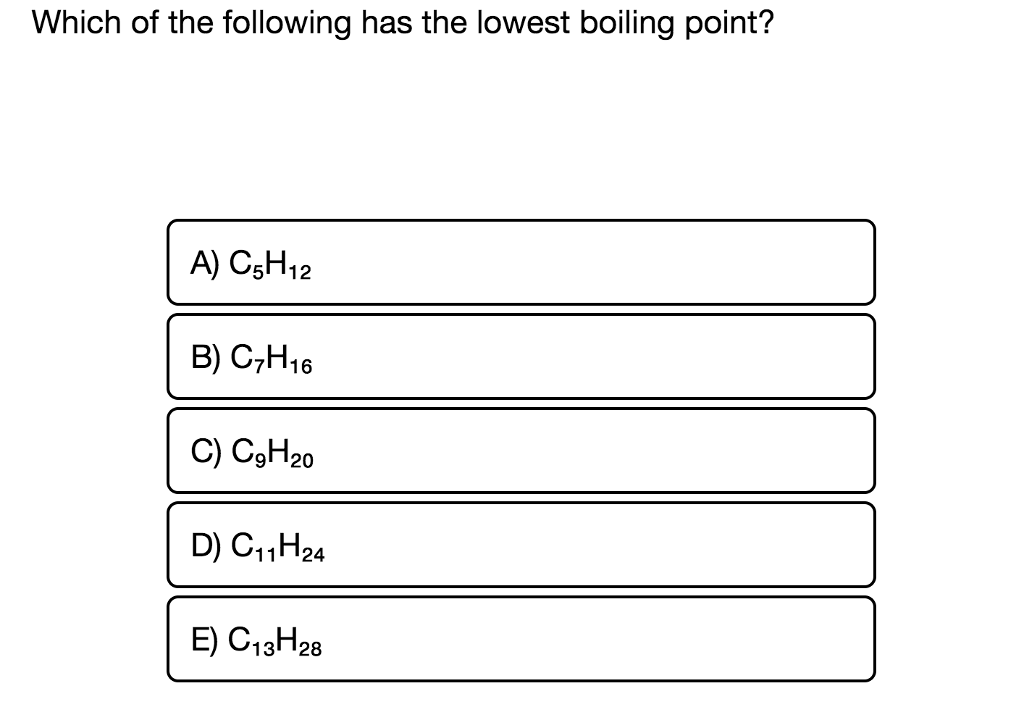 Source: chegg.com
Source: chegg.com
It has been found that helium has the lowest normal boiling point (−268.9 °c) because it has very weak intermolecular attractions. Br2, f2, i2, cl2, answer higher boiling points will correspond to stronger intermolecular forces. Among the following select the alkane that is expected to have lowest boiling point.
 Source: oneclass.com
Source: oneclass.com
If true enter 1 else 0. Hf > hi > hbr > hcl but the important thing to know is not the order itself but why that order happened. Which of the following hydrides has the lowest boiling point h2o h2s h2se.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
Due to least surface area, neopentane has the lowest b.p. The chemical element with the lowest boiling point is helium and the element with the highest boiling point is tungsten. I for glucose is 1.
 Source: bartleby.com
Source: bartleby.com
A) h20 b) n2 c) soz d) h2 e) co2 which one of the following correctly ranks the compounds in order of lowest boiling point to highest boiling point based only in. The midweight alkanes are liquids; At room temperature, the lighter alkanes are gases;
![Solved] Which Compound Has The Lowest Boiling Point? | Course Hero](https://www.coursehero.com/qa/attachment/16046178/ “Solved] Which Compound Has The Lowest Boiling Point? | Course Hero”) Source: coursehero.com
Closed nov 11, 2021 by swamijain. 5)of the following substances, _____ has the highest boiling point. Among the following select the alkane that is expected to have lowest boiling point.
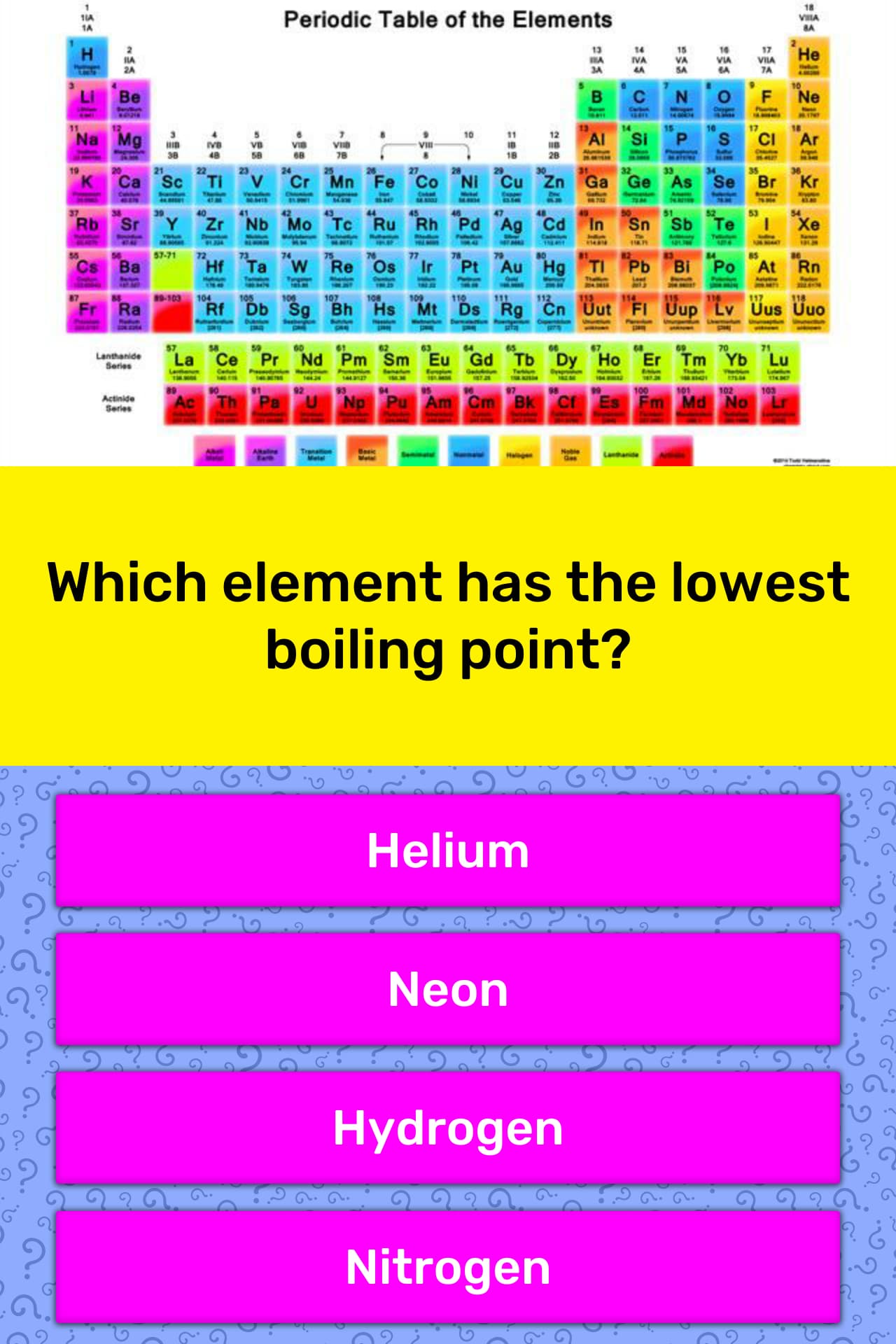 Source: quizzclub.com
Source: quizzclub.com
.fo 9) which of the following alkynes has the lowest boiling point? It has been found that helium has the lowest normal boiling point (−268.9 °c) because it has very weak intermolecular attractions. The magnitude of london dispersion forces decreases with a decrease in molecule size (carbon chain length and molecular surface area).
 Source: studylib.net
Source: studylib.net
If their policy has a deductible of $1,000, what is their. Methanol has strong hydrogen bonds. I will give you a general way that will help you to know not only the below answer but any answer the below picture is the structure of pentane they are all aligned in the parent chain which is a very strong bond and donot dissociate properly another picture is the picture of.
 Source: chegg.com
Source: chegg.com
Hence it has the lowest boiling point. I for ethylene glycol is 1. 120 rows for chemistry students and teachers:
 Source: study.com
Source: study.com
I for glucose is 1. But due to high symmetry it has the highest m.p. I for glucose is 1.
 Source: chegg.com
Source: chegg.com
Boiling points are a measure of intermolecular forces. Which alcohols have the lowest boiling point? Br2, f2, i2, cl2, answer higher boiling points will correspond to stronger intermolecular forces.
 Source: youtube.com
Source: youtube.com
I for ethylene glycol is 1. Let�s go to the basics; So the highest boiling point will be the one that has the highest i*m which is na2so4.
 Source: youtube.com
Source: youtube.com
Methanol has strong hydrogen bonds. And the heavier alkanes are solids, or tars. Halogen literally means �salt producing� since thes.
 Source: doubtnut.com
Source: doubtnut.com
It will have the next highest boiling point. The boiling point of a liquid at 1 atm is also known as the normal boiling point. Heliumthe chemical element with the lowest boiling point is helium and the element with the highest boiling point is tungsten.
Also Read :