The more sphere like the molecule, the lower its surface area will be and the fewer intermolecular van der waals interactions will operate. All the given compounds are alkanes so the only present imf is van der waals forces.
Which Of The Following Has The Highest Boiling Point. Ans.(a) in general conception, as the branching increases packing of the molecules in the crystals becomes less close and have melting point decreases accordingly. What type of deviation is shown by minimum boiling azeotropes? As we known greater the value of van’t hoff factor higher will be the elevation in bonding point and hence higher will be the boiling point of solution. ↑ intermolecular force → ↑ boiling point;
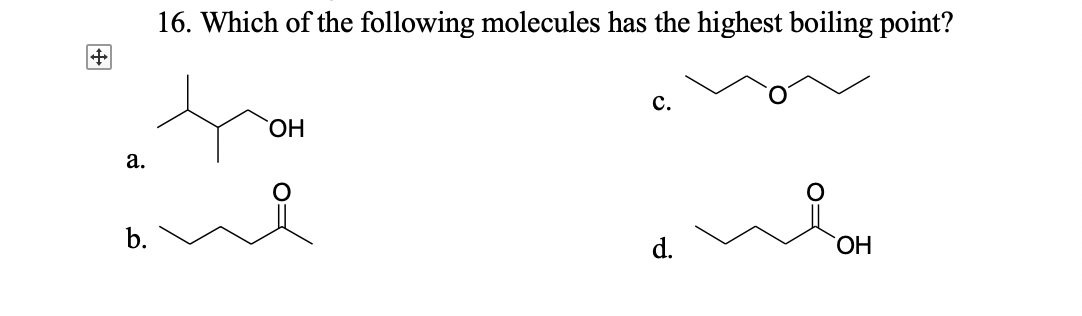 Answered: 16. Which Of The Following Molecules… | Bartleby From bartleby.com
Answered: 16. Which Of The Following Molecules… | Bartleby From bartleby.com
Related Post Answered: 16. Which Of The Following Molecules… | Bartleby :
Neopentane it has the lowest boiling point among the three but has highest melting point. In the case of (iii) i.e. The vander waals dispersion forces increase as the length of the hydrocarbon chain increases. Hcl will have a lower boiling point than hf since f is more electronegative than cl and possess a greater degree of hydrogen bonding.
Correspondingly, i2 will have the highest boiling point and f2 will have the lowest boiling point.
Which compound has the highest boiling point? Sodium chloride has boiling point 1413 °c. We are asked which of the following compounds has the highest boiling point. The vander waals dispersion forces increase as the length of the hydrocarbon chain increases. Of the following, _____ has the highest boiling point. (ii asked apr 9, 2020 in chemistry by prishabasu ( 96.1k points)
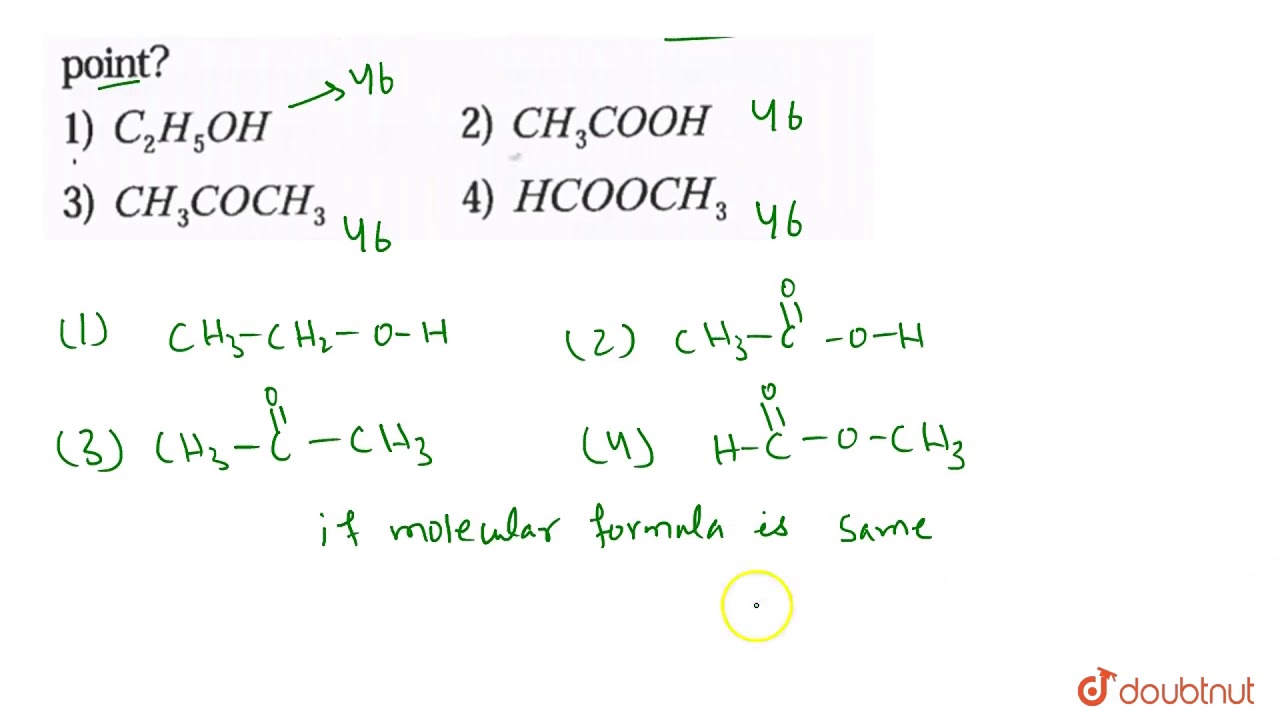 Source: youtube.com
Source: youtube.com
Isopentane, it has boiling point less than (i) but more than (iii). H2o has the highest boiling point out of. Answered by wiki @ 03/12/2021.
 Source: toppr.com
Source: toppr.com
Which of the following has the highest boiling point? Which compound has the highest boiling point? They increase with the following properties:
 Source: youtube.com
Source: youtube.com
The boiling point is the temperature at which a substance will change from the liquid phase to the gas phase, so compounds with stronger intermolecular forces will have higher boiling points. Selected jun 5, 2019 by mihika sahu. Which substance has the highest/lowest melting/boiling point, etc.

So, $ch _{3} ch _{2} ch _{2} ch _{2} cl$ has highest boiling point. So i2 has the strongest forces, and f2 will have the weakest. Answered by wiki @ 03/12/2021.
 Source: edurev.in
Source: edurev.in
The boiling point is the temperature at which a substance will change from the liquid phase to the gas phase, so compounds with stronger intermolecular forces will have higher boiling points. Hence 1.0mna2so4 has highest value of bonding point. What type of deviation is shown by minimum boiling azeotropes?
 Source: doubtnut.com
Source: doubtnut.com
Hcl will have a lower boiling point than hf since f is more electronegative than cl and possess a greater degree of hydrogen bonding. Higher boiling points will correspond to stronger intermolecular forces. On the basis of bonding, the compound which has highest boiling point is sodium chloride.
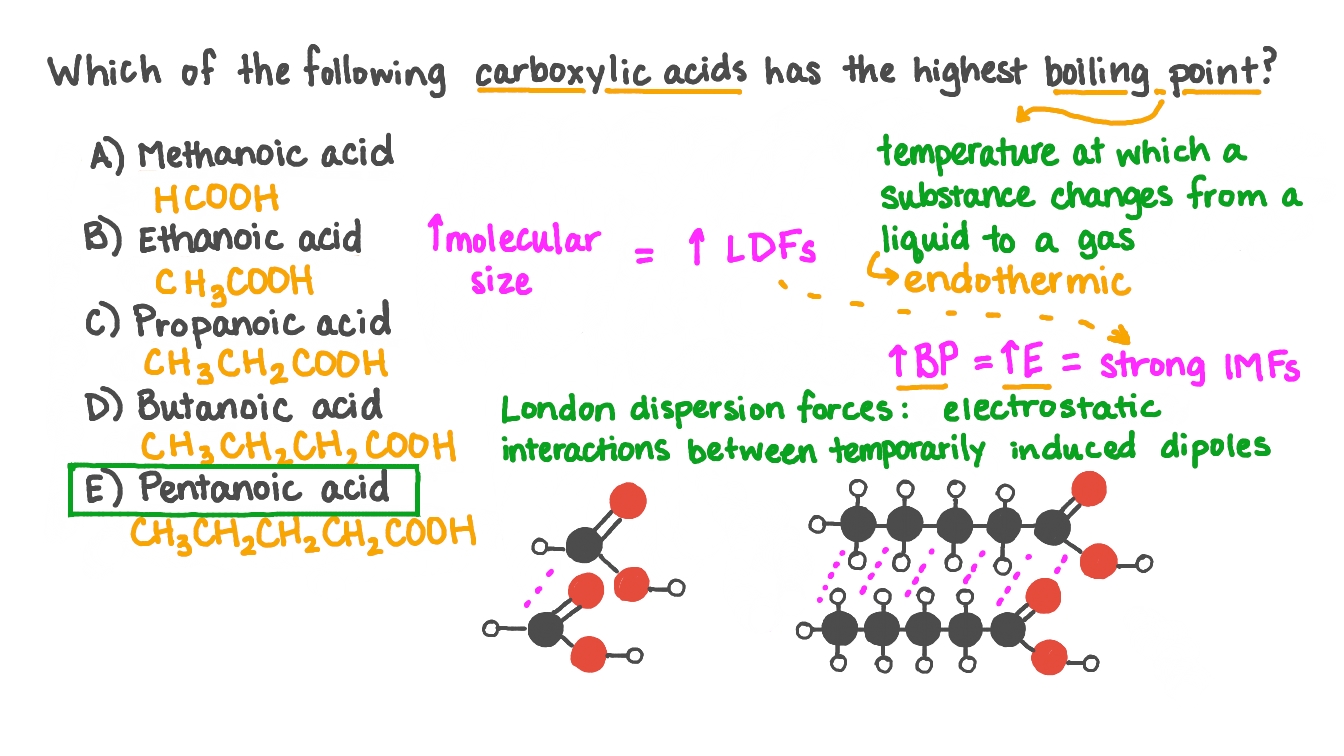 Source: nagwa.com
Source: nagwa.com
The boiling point of butane is close to 0 degrees celsius, whereas the higher boiling point of butanone (79.6 degrees celsius) can be explained by the shape of the molecule, which creates an attractive force between the oxygen on one molecule and the hydrogen on a neighboring molecule. On the basis of bonding, the compound which has highest boiling point is sodium chloride. However h2o unexpectedly has highest boiling point due to presence of hydrogen bonding.

Hcl will have a lower boiling point than hf since f is more electronegative than cl and possess a greater degree of hydrogen bonding. Which of the following has the highest boiling point? F2, cl2, br2, i2 which substance has the highest/lowest melting/boiling point, etc.
 Source: bartleby.com
Source: bartleby.com
Bigger molecules will have stronger london dispersion forces. The vander waals dispersion forces increase as the length of the hydrocarbon chain increases. Isopentane, it has boiling point less than (i) but more than (iii).
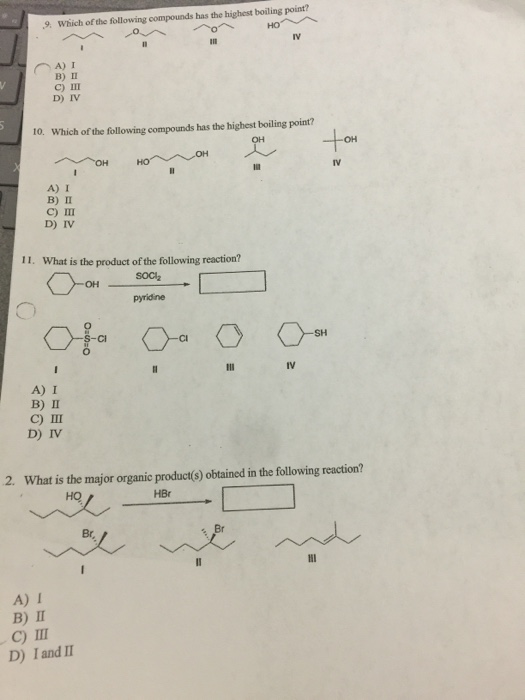 Source: chegg.com
Source: chegg.com
Which following has highest bp? As we known greater the value of van’t hoff factor higher will be the elevation in bonding point and hence higher will be the boiling point of solution. Correspondingly, i2 will have the highest boiling point and f2 will have the lowest boiling point.
 Source: meritnation.com
Source: meritnation.com
Water has the highest boiling point because water is a strong dipole and the molecules are interconnected by hydrogen bonds. (b) as the branching increases,. The boiling point is the temperature at which a substance will change from the liquid phase to the gas phase, so compounds with stronger intermolecular forces will have higher boiling points.
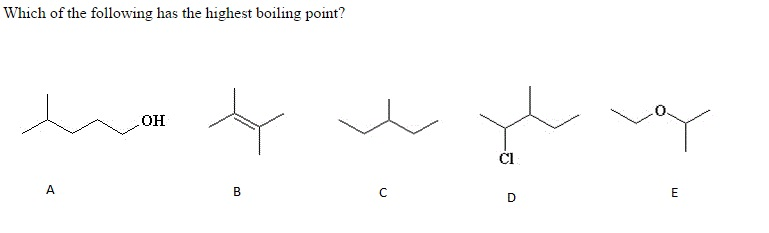 Source: chegg.com
Source: chegg.com
H2o has the highest boiling point out of. In the case of (iii) i.e. All the given compounds are alkanes so the only present imf is van der waals forces.
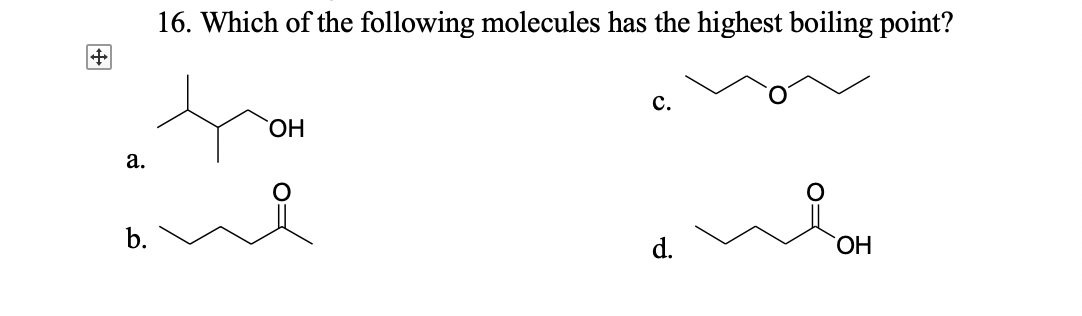 Source: bartleby.com
Source: bartleby.com
Size (the heavier, the stronger the force) Glucose usually melts at 146 °c. Which substance has the highest/lowest melting/boiling point, etc.
 Source: ask.learncbse.in
Source: ask.learncbse.in
0.1 m aqueous solution of ferric chloride has the highest boiling point. So, $ch _{3} ch _{2} ch _{2} ch _{2} cl$ has highest boiling point. Answer:hf will have the highest boiling point.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
Consider the following statements (i) in a group of isomeric acyclic compounds, normal compound always has the highest boiling and melting points. Which following has highest bp? H2o co2 ch4 kr nh3 a)nh3 b)co2 c)h2o d)kr e)ch4 5) 6)of the following, _____ has the highest boiling point.
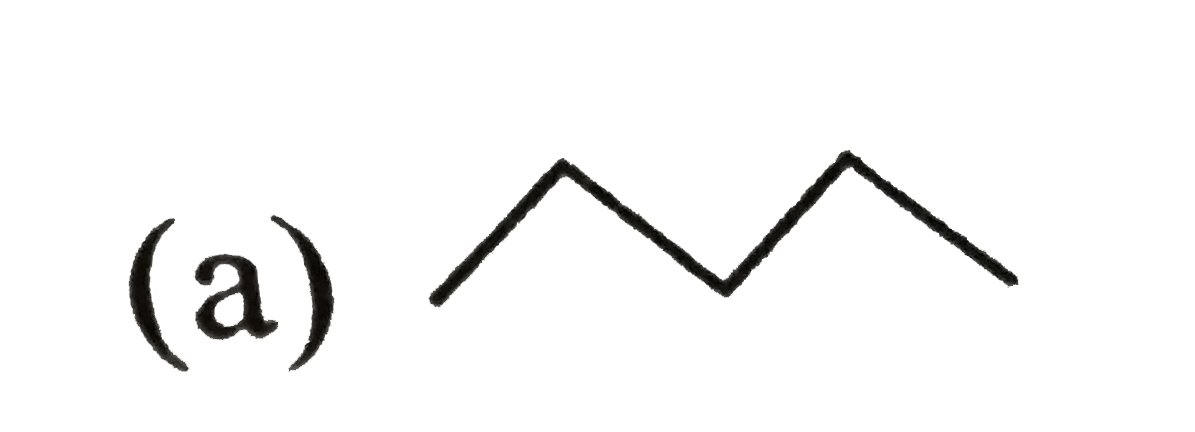 Source: sarthaks.com
Source: sarthaks.com
We are asked which of the following compounds has the highest boiling point. Therefore, hf will have the strongest intermolecular forces and thus the highest boiling point. Compounds that can hydrogen bond will have higher boiling points than compounds that can only interact through london dispersion forces.
 Source: neetlab.com
Source: neetlab.com
Ans.(a) in general conception, as the branching increases packing of the molecules in the crystals becomes less close and have melting point decreases accordingly. Isopentane, it has boiling point less than (i) but more than (iii). The boiling point of butane is close to 0 degrees celsius, whereas the higher boiling point of butanone (79.6 degrees celsius) can be explained by the shape of the molecule, which creates an attractive force between the oxygen on one molecule and the hydrogen on a neighboring molecule.
 Source: clutchprep.com
Source: clutchprep.com
Sodium chloride has boiling point 1413 °c. The boiling point is the temperature at which a substance will change from the liquid phase to the gas phase, so compounds with stronger intermolecular forces will have higher boiling points. So the highest boiling point will be the one that has the highest i*m which is na2so4.
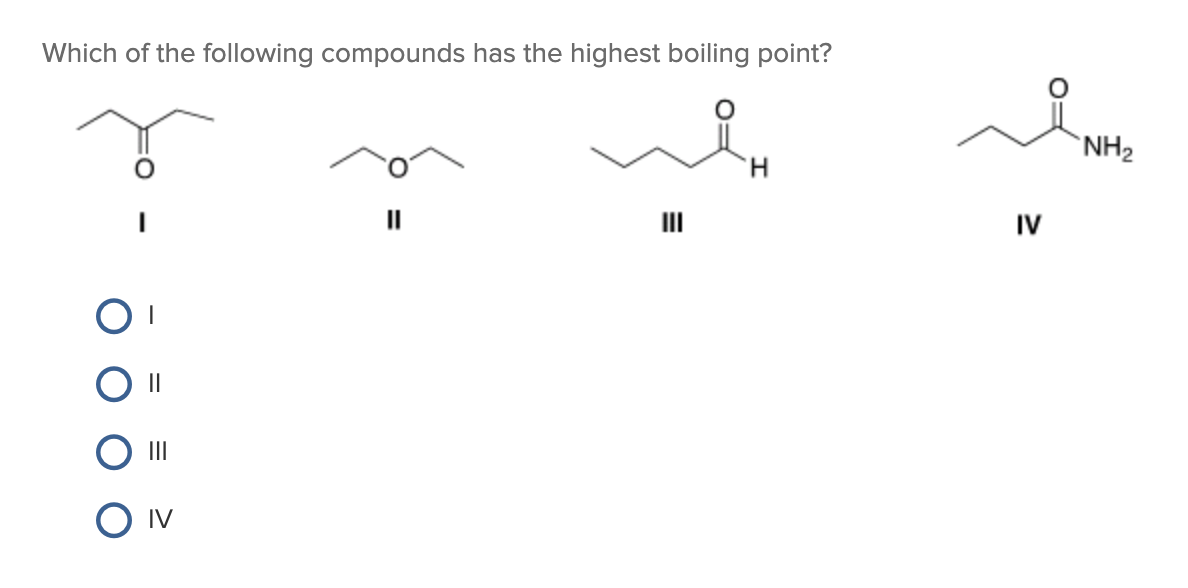 Source: chegg.com
Source: chegg.com
Selected jun 5, 2019 by mihika sahu. Hence c4 h9 clstraight chain molecule with the highest number of carbons has the highest boiling point. The more sphere like the molecule, the lower its surface area will be and the fewer intermolecular van der waals interactions will operate.
Also Read :