The number of electrons per shell of phosphorus is 2, 8, 5. Therefore, the valence electrons of neon(ne) are eight.
Which Of The Following Has Eight Valence Electrons. 2) which of the following does not have eight valence electrons? Configuration of the valence shell: Compound formation of oxygen by valence electrons. This valence electron participates in the formation of bonds with atoms of other elements.
 Solved N Which Of The Following Has Eight Valence Electrons? | Chegg.com From chegg.com
Solved N Which Of The Following Has Eight Valence Electrons? | Chegg.com From chegg.com
Related Post Solved N Which Of The Following Has Eight Valence Electrons? | Chegg.com :
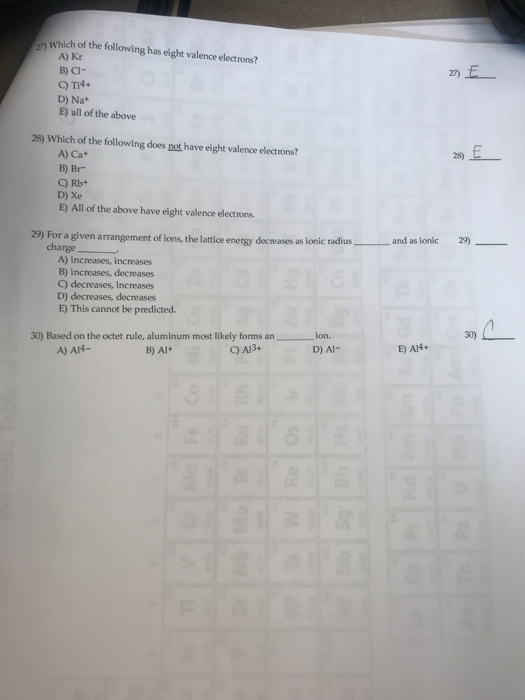
Atoms with 8 electrons in their valence shell have completely filled last orbitals and are therefore the most stable, as their electronic configuration is similar to that of the closest noble gas. The outermost orbit of the atom cannot have less than 8 electrons. 1) which of the following has eight valence electrons? If an orbit has 8 electrons, then it will be the outermost orbit of that atom.
If an orbit has 8 electrons, then it will be the outermost orbit of that atom.
Examples include neon (ne), argon (ar), and krypton (kr). If an orbit has 8 electrons, then it will be the outermost orbit of that atom. The columns that were set up to group elements by similar chemical properties turn out to be the exact same columns defined by the number of valence electrons. Configuration of the valence shell: Compound formation of oxygen by valence electrons. All there are ____ valence electrons in the lewis structure of ch_3, ch_2cl.
Examples include hydrogen (h), lithium (li), and sodium (na). Now, magnesium, mg , has 2 valence electrons. 10 in the lewis symbol for a sulfur atom, there are _____ paired and _____ unpaired electrons.

Now, magnesium, mg , has 2 valence electrons. The number 8 is a lucky number, according to many asian cultures. Therefore, the valence electrons of neon(ne) are eight.
 Source: khanacademy.org
Source: khanacademy.org
- lattice energy is _____. The atoms� valence electrons are shared between. The five elements (noble gases) neon (ne), argon (ar), krypton (kr), xenon (xe) and radon (rn) have 8 valence electrons.
 Source: msrblog.com
Source: msrblog.com
Therefore, the valence electrons of neon(ne) are eight. If an orbit has 8 electrons, then it will be the outermost orbit of that atom. But for most of the transition and inner transition elements, the valence electrons are the electrons present in the shells outside the noble gas core.
 Source: chegg.com
Source: chegg.com
The columns that were set up to group elements by similar chemical properties turn out to be the exact same columns defined by the number of valence electrons. The number of electrons per shell of phosphorus is 2, 8, 5. The electron configuration of neon(ne) shows that the last shell of neon has eight electrons.
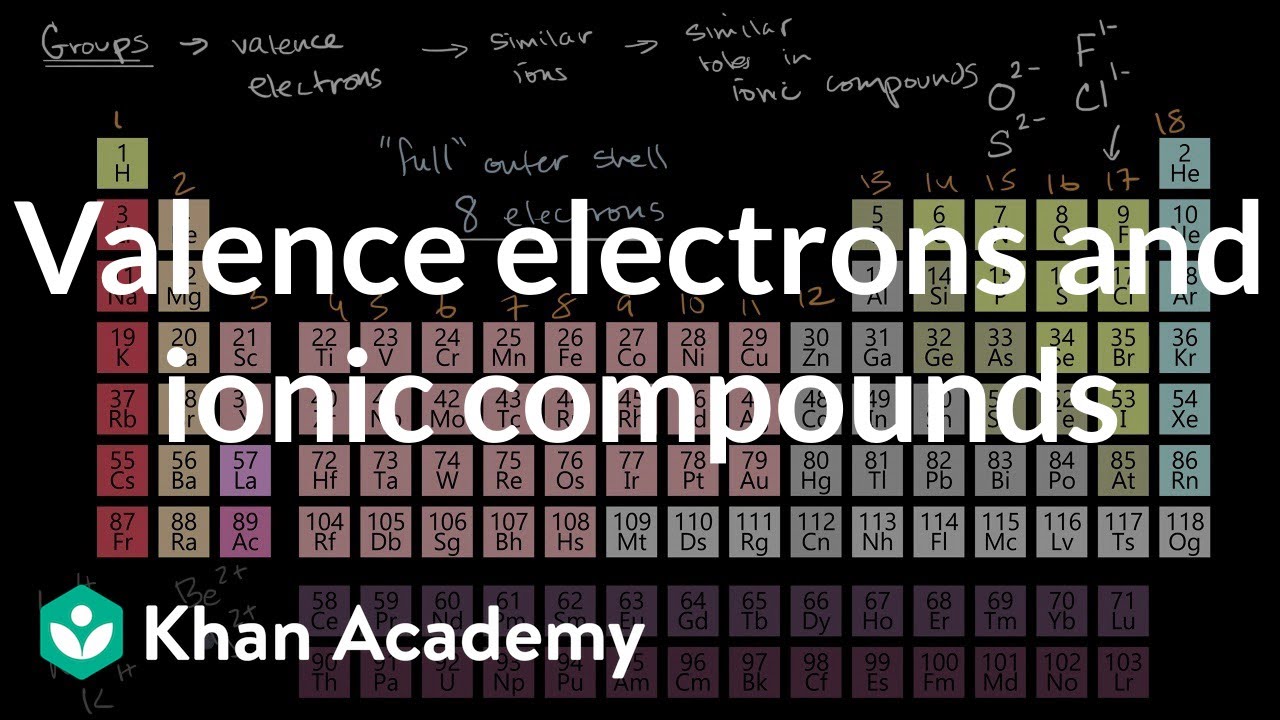 Source: khanacademy.org
Source: khanacademy.org
Atoms with 8 electrons in their valence shell have completely filled last orbitals and are therefore the most stable, as their electronic configuration is similar to that of the closest noble gas. It has only one electron in its valence shell. D) the oxygen no longer has eight valence electrons.
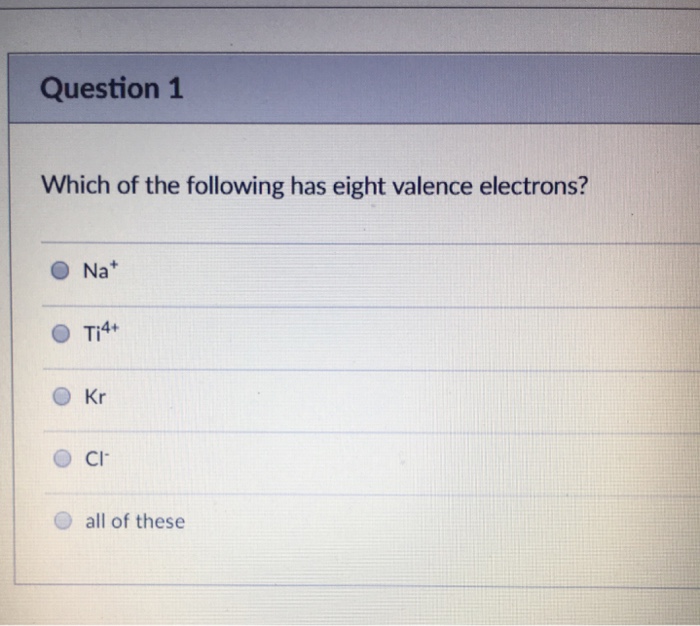
Does magnesium have 8 valence electrons? Helium has two electrons in its outermost shell and all other elements have atoms with eight electrons in the outermost shell. 10 in the lewis symbol for a sulfur atom, there are _____ paired and _____ unpaired electrons.
 Source: chegg.com
Source: chegg.com
A) both bonding electrons come from the oxygen atom. B) it forms an especially strong bond. C) the electrons are equally shared.
 Source: chegg.com
Source: chegg.com
Examples include neon (ne), argon (ar), and krypton (kr). It has only one electron in its valence shell. The number of electrons per shell of phosphorus is 2, 8, 5.

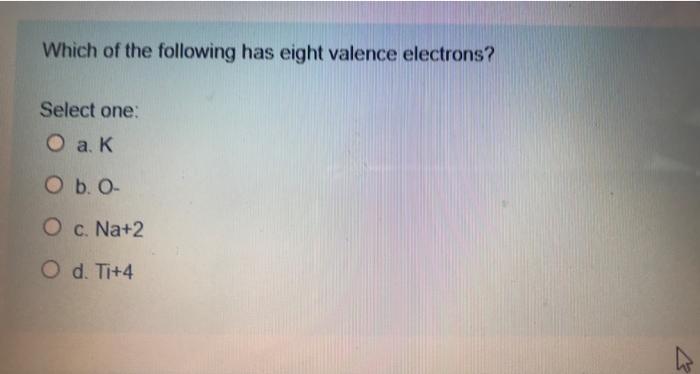
- which of the following has eight valence electrons? Cl is right next to argon. Does magnesium have 8 valence electrons?
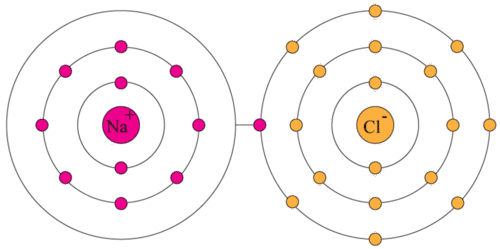
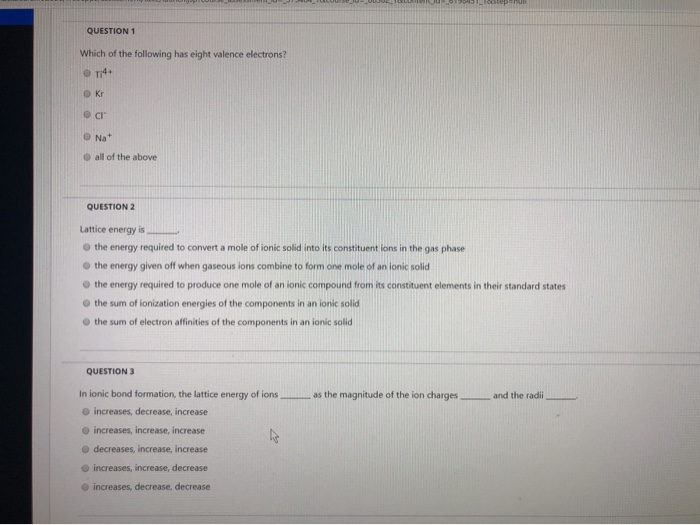
Which of the following has eight valence electrons? These questions are based on the attached photo. The atoms� valence electrons are shared between.
 Source: oneclass.com
Source: oneclass.com
Examples include hydrogen (h), lithium (li), and sodium (na). D) the oxygen no longer has eight valence electrons. All there are ____ valence electrons in the lewis structure of ch_3, ch_2cl.

- which of the following has eight valence electrons? The number of electrons per shell of phosphorus is 2, 8, 5. 1 show answers another question on chemistry.
 Source: itprospt.com
Source: itprospt.com
Valence electrons are transferred from one atom to the other. Atoms with 8 electrons in their valence shell have completely filled last orbitals and are therefore the most stable, as their electronic configuration is similar to that of the closest noble gas. Now, magnesium, mg , has 2 valence electrons.

The only way for magnesium to accomplish this is to give up its two valence electrons and have one of its filled energy levels become the valence shell, i.e. 10 in the lewis symbol for a sulfur atom, there are _____ paired and _____ unpaired electrons. But for most of the transition and inner transition elements, the valence electrons are the electrons present in the shells outside the noble gas core.
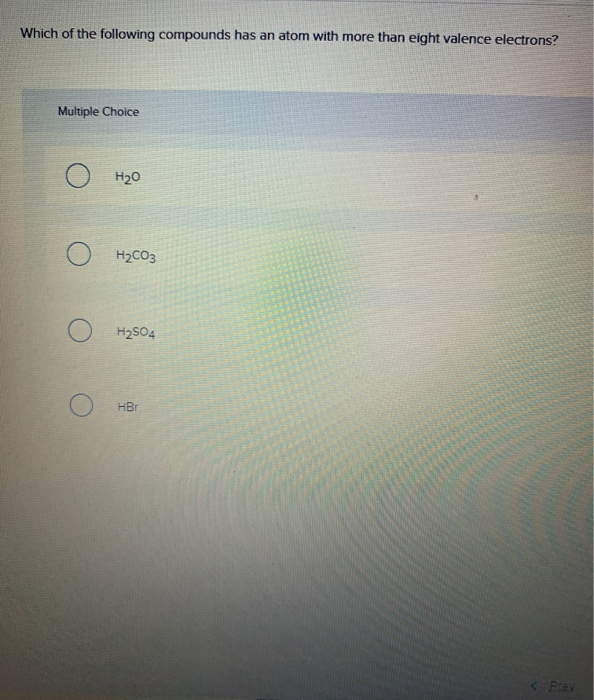 Source: chegg.com
Source: chegg.com
10 in the lewis symbol for a sulfur atom, there are _____ paired and _____ unpaired electrons. The last shell after the electron configuration is called the valence shell (orbit). 3s2 3p6 total number of valence electrons = 8.
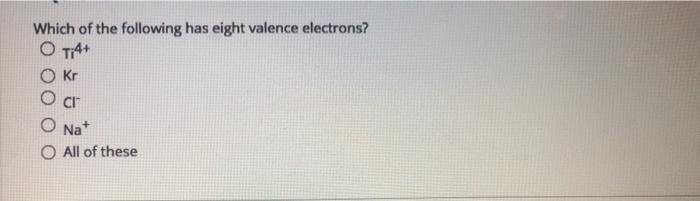
The electron configuration of neon(ne) shows that the last shell of neon has eight electrons. 10 in the lewis symbol for a sulfur atom, there are _____ paired and _____ unpaired electrons. Helium has two electrons in its outermost shell and all other elements have atoms with eight electrons in the outermost shell.
 Source: chegg.com
Source: chegg.com
Examples include neon (ne), argon (ar), and krypton (kr). But for most of the transition and inner transition elements, the valence electrons are the electrons present in the shells outside the noble gas core. The number of electrons per shell of phosphorus is 2, 8, 5.
 Source: chegg.com
Source: chegg.com
Any element in group 18 has eight valence electrons (except for helium, which has a total of just two electrons). 1 📌📌📌 question which of the following has eight valence electrons? Which of the following has eight valence electrons?
 Source: chegg.com
Source: chegg.com
What elements have 8 valence electrons? Any element in group 18 has eight valence electrons (except for helium, which has a total of just two electrons). To attain 8 electrons in its valence shell, it should lose two electrons from its valence shell and attain the configuration [he] 2s2 2p6 3s2 3p6.
Also Read :