Dipole moment of ch4 is zero. So you know in this case the boy moment that a positive direction and our cancel with each other.
Which Of The Following Has A Zero Dipole Moment. Dipole moment of ch4 is zero. Due to the linear structure of the molecule, Bef2 has a linear shape. Does ch4 have a dipole moment?
 Which Of The Following Has Zero Dipole Moment? From doubtnut.com
Which Of The Following Has Zero Dipole Moment? From doubtnut.com
Related Post Which Of The Following Has Zero Dipole Moment? :
Are solved by group of students and teacher of jee, which is also the largest student community of jee. The two bond dipole moments (c=o) are equal and opposite in direction. Which one of the following has zero dipole moment? Using periodic trends, arrange the following molecules in order of increasing dipole moment:
So, in (bf_{3}) net dipole moment is zero.
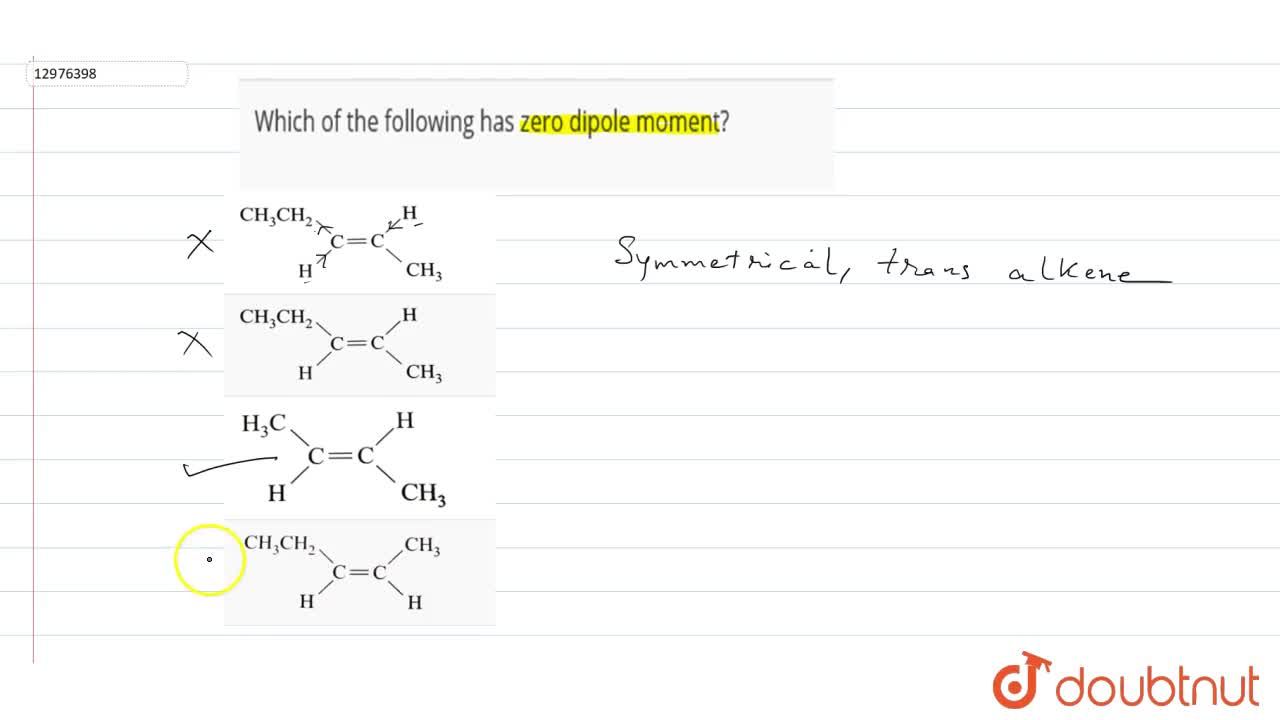
There exist two individual bond dipole moments, which cancel each other resulting in the net dipole moment zero. The ch4 is tetrahedral in shape thus each bond pair are at equal distance that is they are symmetrically arranged hence each dipole moment of bond balance each other. (1) nh 3 (2) h 2 o (3) bcl 3 (4) so 535. Methane, ch4, does not have a dipole moment. How many isomers of dichloroethylene have a zero dipole moment??therefore, only one isomer of dichloroethylene has a zero dipole moment.what compounds have a dipole moment of zero?in the triatomic co2 (carbon dioxide) molecule, the dipole moment is zero. In beryllium fluoride molecule, the dipole moment is zero.
 Source: youtube.com
Source: youtube.com
Determine the lewis structure of each compound (co2, nh3, h2o and hf) and figure out which has zero dipole moment. What is a 0 dipole moment? The order of dipole moment is given below.
 Source: goiit.com
Source: goiit.com
So you know in this case the boy moment that a positive direction and our cancel with each other. Which of the following has zero dipole moment bcl3? Draw the lewis structure for the molecule.
 Source: youtube.com
Source: youtube.com
Are solved by group of students and teacher of jee, which is also the largest student community of jee. To do so, we first need to do the following steps: H 2 o have bent structure in which the two o − h bonds are oriented at an angle of 104.5 ∘, so water have a net dipole moment whereas b e f 2 have linear geometry, so the dipole moment of one bond is cancelled by another bond, so it have zero dipole moment.
 Source: doubtnut.com
Source: doubtnut.com
Using periodic trends, arrange the following molecules in order of increasing dipole moment: Thus, it will not have any net dipole moment. Therefore they cancel each other resulting in zero net dipole moment.
 Source: doubtnut.com
Source: doubtnut.com
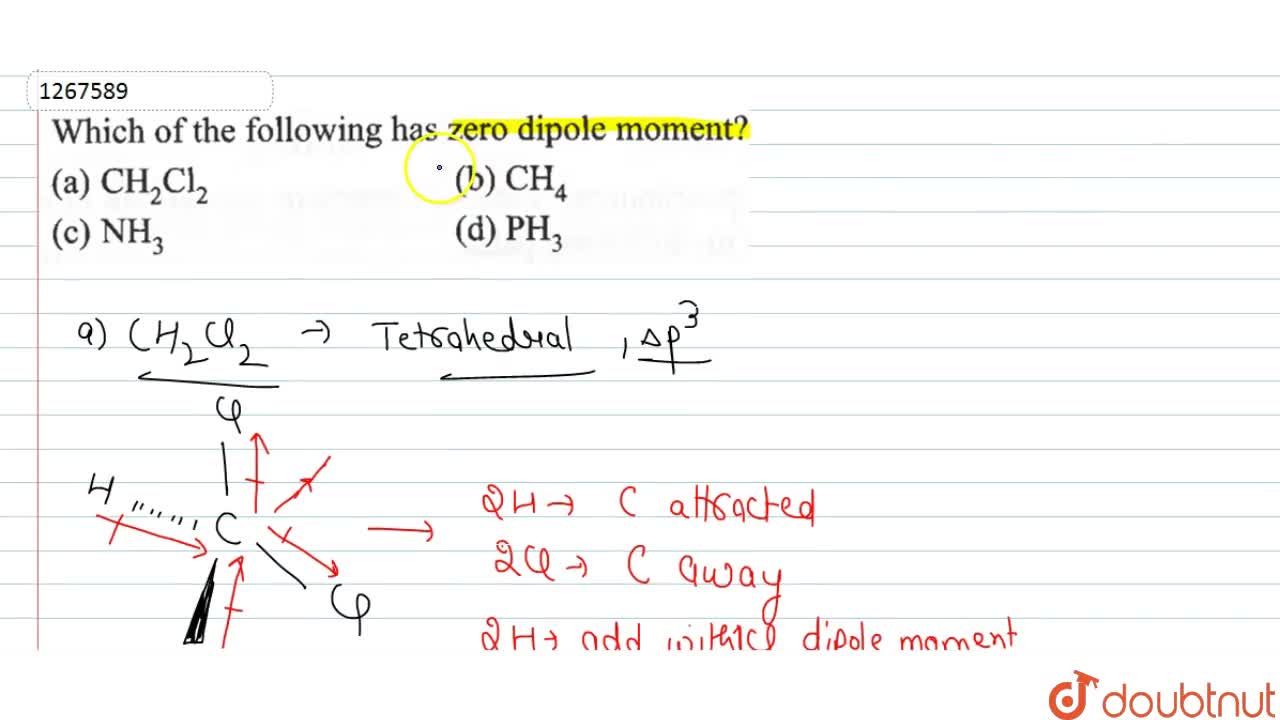
H 2 o have bent structure in which the two o − h bonds are oriented at an angle of 104.5 ∘, so water have a net dipole moment whereas b e f 2 have linear geometry, so the dipole moment of one bond is cancelled by another bond, so it have zero dipole moment. Check answer and solution for above question from che (a) ch2cl2 (b) nh3 (c) ch4 (d) ph3.
 Source: toppr.com
Source: toppr.com
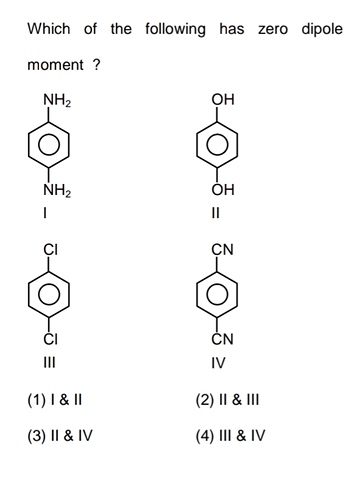
Asked nov 29, 2019 in chemistry by hitheshkumar (85.2k points) closed dec 16, 2021 by hitheshkumar. Determine the central atom in the molecule. Nitrogen and hydrogen have a huge difference in electronegativity, and because of the geometry, the moments are not canceled, and the dipole moment is different from 0.
 Source: meritnation.com
Source: meritnation.com
Check answer and solution for above question from che H 2 o have bent structure in which the two o − h bonds are oriented at an angle of 104.5 ∘, so water have a net dipole moment whereas b e f 2 have linear geometry, so the dipole moment of one bond is cancelled by another bond, so it have zero dipole moment. Dipole moment of ch4 is zero.
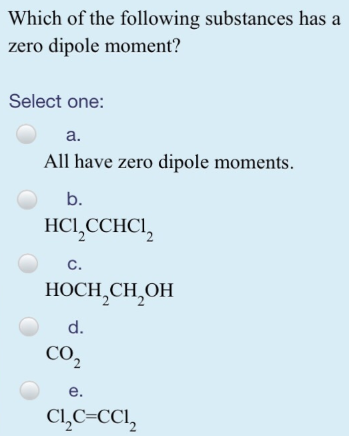 Source: clutchprep.com
Source: clutchprep.com
Sum of bond dipole moments will give the net dipole moment for a molecule. Co2 is a linear symmetrical molecule. Due to the linear structure of the molecule,
 Source: sahay.guru
Source: sahay.guru
Are solved by group of students and teacher of jee, which is also the largest student community of jee. Determine the lewis structure of each compound (co2, nh3, h2o and hf) and figure out which has zero dipole moment. Due to the linear structure of the molecule,
 Source: youtube.com
Source: youtube.com
Which of the following fluoride of xenon has zero dipole moment? Methane, ch4, does not have a dipole moment. The order of dipole moment is given below.
 Source: toppr.com
Source: toppr.com
No side of the molecule has more negative or positive charge than another side, and so the molecule is nonpolar. This is because this cultural structure of trans two baldini�s moving is see you will want to see parents. Bf3 has zero dipole moment because all vector cancel the effect of each other.

Co2 has zero dipole moment due to its linear shape. Co2 has zero dipole moment due to its linear shape. The central atom, n, will have a lone pair of electrons, so the geometry here will be angular.
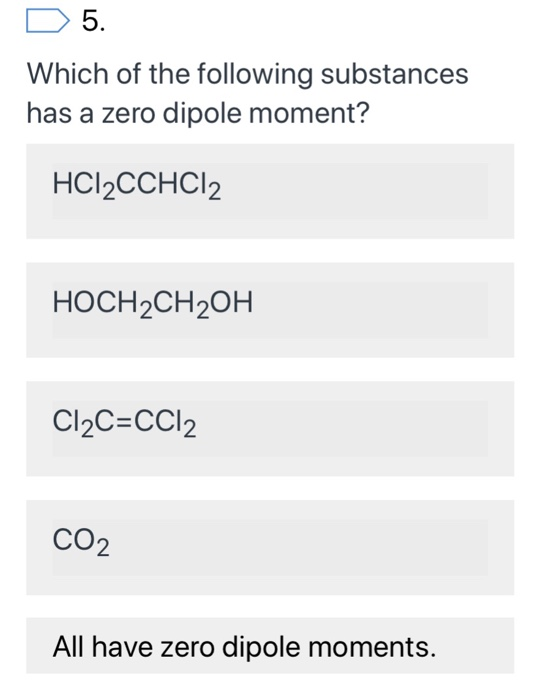 Source: chegg.com
Source: chegg.com
That�s the age, three age and ch three. To do so, we first need to do the following steps: Among nh3,h2o and chcl3, water has highest dipole moment because all the vector is added up to give large dipole moment.
 Source: youtube.com
Source: youtube.com
Using periodic trends, arrange the following molecules in order of increasing dipole moment: The two bond dipole moments (c=o) are equal and opposite in direction. Which of the following has zero dipole moment?
 Source: tardigrade.in
Source: tardigrade.in
The arrangement of lone pair as well as bond pairs is. This is because in bef2 molecule, the bond dipole moments are equal in magnitude and opposite in direction. Which of the following has zero dipole moment bcl3?

So you know in this case the boy moment that a positive direction and our cancel with each other. (bf_3) have three bonds, and they are significantly polar, but they are symmetrically arranged around the central boron atom. The ch4 is tetrahedral in shape thus each bond pair are at equal distance that is they are symmetrically arranged hence each dipole moment of bond balance each other.
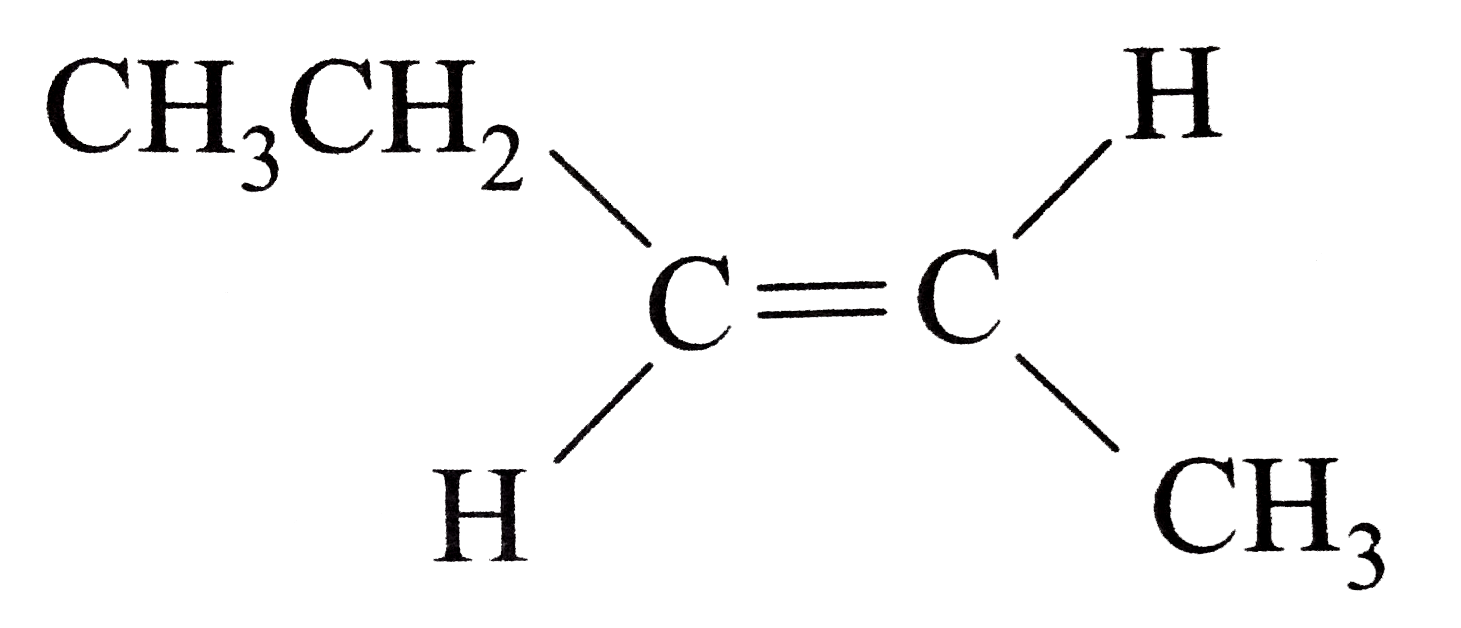 Source: sarthaks.com
Source: sarthaks.com
The order of dipole moment is given below. Methane, ch4, does not have a dipole moment. Sum of bond dipole moments will give the net dipole moment for a molecule.

(1) nh 3 (2) h 2 o (3) bcl 3 (4) so 535. N f 3 also has dipole moment in the opposite direction as compared to n h3. The dipole moment of the trans compounds are zero as they cancel out each other.
 Source: toppr.com
Source: toppr.com
There exist two individual bond dipole moments, which cancel each other resulting in the net dipole moment zero. (a) ch2cl2 (b) nh3 (c) ch4 (d) ph3. Which one of the following molecules has a zero dipole moment?
 Source: meritnation.com
Source: meritnation.com
A molecule xy2 contains two s, two p bonds and one lone pair of electrons in the valence shell of x. This is because in bef2 molecule, the bond dipole moments are equal in magnitude and opposite in direction. Methane, ch4, does not have a dipole moment.
Also Read :