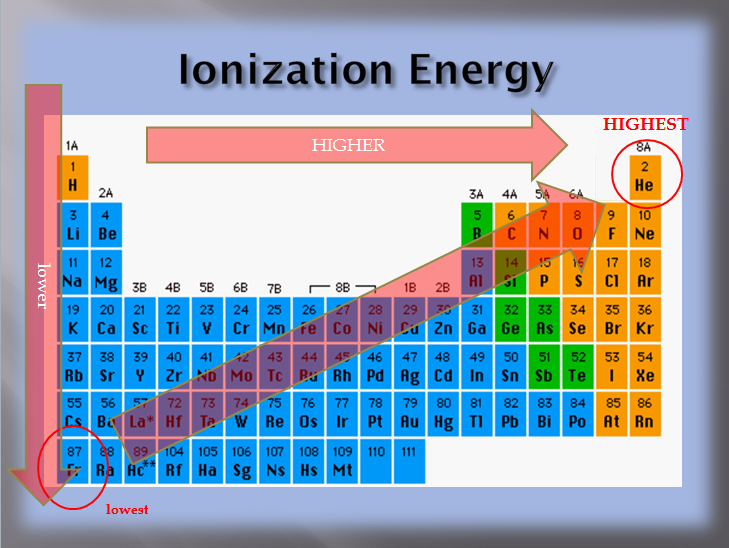
So, sulphur has the lowest ionization potential among all four. Cesiumthe element with the lowest ionization energy is cesium (cs).
Which Of The Following Elements Has The Lowest Ionization Energy. Cesium has atomic number 55 and is in the fifth row of the periodic table. And the element which has the lowest ionization energy is caesium in 3.8939 ev. Why does boiling point decrease across a period? Compared to the ionization energy of a magnesium atom, the ionization energy of a calcium atom is smaller, this is primarily because the calcium atom:
 Which Of The Following Elements Will Have The Lowest First Ionisation Energy? - Youtube From youtube.com
Which Of The Following Elements Will Have The Lowest First Ionisation Energy? - Youtube From youtube.com
Related Post Which Of The Following Elements Will Have The Lowest First Ionisation Energy? - Youtube :
Sindh mcqs, 12th class mcqs, chemistry mcqs, periodic classification mcqs, na , f , i , cs Group 1 of the periodic table features metals whose valence electron singly occupies the outermost shell, the valence shell of the given atoms. Among the following, which element has the lowest ionization energy? The element with the lowest ionization energy is cesium (cs).
Ionization energy, also called ionization potential, in chemistry and physics, the amount of energy required to remove an.
The electron configuration of the ions will match that of a noble gas. Thus, helium has the largest first ionization energy, while francium has one of the lowest. I am not a gas at room temperature. Group 1 of the periodic table features metals whose valence electron singly occupies the outermost shell, the valence shell of the given atoms. The group of elements which have the lowest ionization energy are the alkali metals. (a) he (b) ne (c) ar (d) kr (e) xe 7.
 Source: quora.com
Source: quora.com
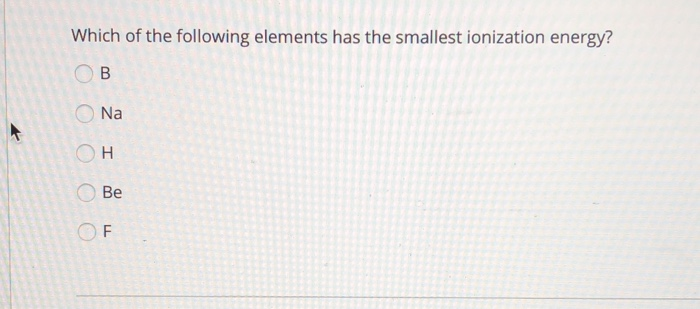
Therefore, option (c) sulphur is correct. Mg2+ would be the smaller ion this is because each ion has the same number of electrons however mg2+ has a greater number of protons and therefore is more charge dense and the outer electrons feel a greater pull from the nucleus. What is ionization energy in simple words?
 Source: chegg.com
Source: chegg.com
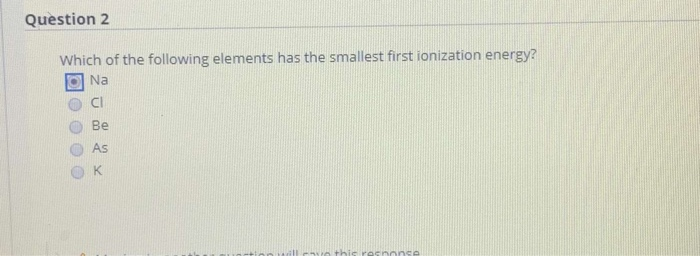
Now in the case of na and mg ,na has lower ionization energy and mg has higher. _____ of the following elements has the lowest first ionization energy? Thus, helium has the largest first ionization energy, while francium has one of the lowest.
 Source: chegg.com
Source: chegg.com
Ionization energy increases going along in a period which begins at minimum for alkali metals and ends at maximum for noble gases. Thus, helium has the largest first ionization energy, while francium has one of the lowest. Thus, helium has the largest first ionization energy, while.
 Source: toppr.com
Source: toppr.com
After removing this electron from 2s orbital it will attain the stable electronic configuration of helium hence the third ionization energy of boron is lowest. Ionization energy value decreases from top to bottom in a group because the shielding effect in atoms increases as we move down a group. Cesiumthe element with the lowest ionization energy is cesium (cs).

My first ionization energy is greater than that of iodine. 104 rows you can print the list of elements by hitting the print button below. Ionization energy, also called ionization potential, in chemistry and physics, the amount of energy required to remove an.
 Source: quizlet.com
Source: quizlet.com
Ionization energy, also called ionization potential, in chemistry and physics, the amount of energy required to remove an. From lowest energy to highest energy which of the following correctly orders the different list the following atoms in order of increasing ionization energy: Li, na, c, o, f.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
Cl, p, si, mg, na. The electron configuration of the ions will match that of a noble gas. Because after removing the two electron its electronic configuration have become 1s2,2s1.
 Source: youtube.com
Source: youtube.com
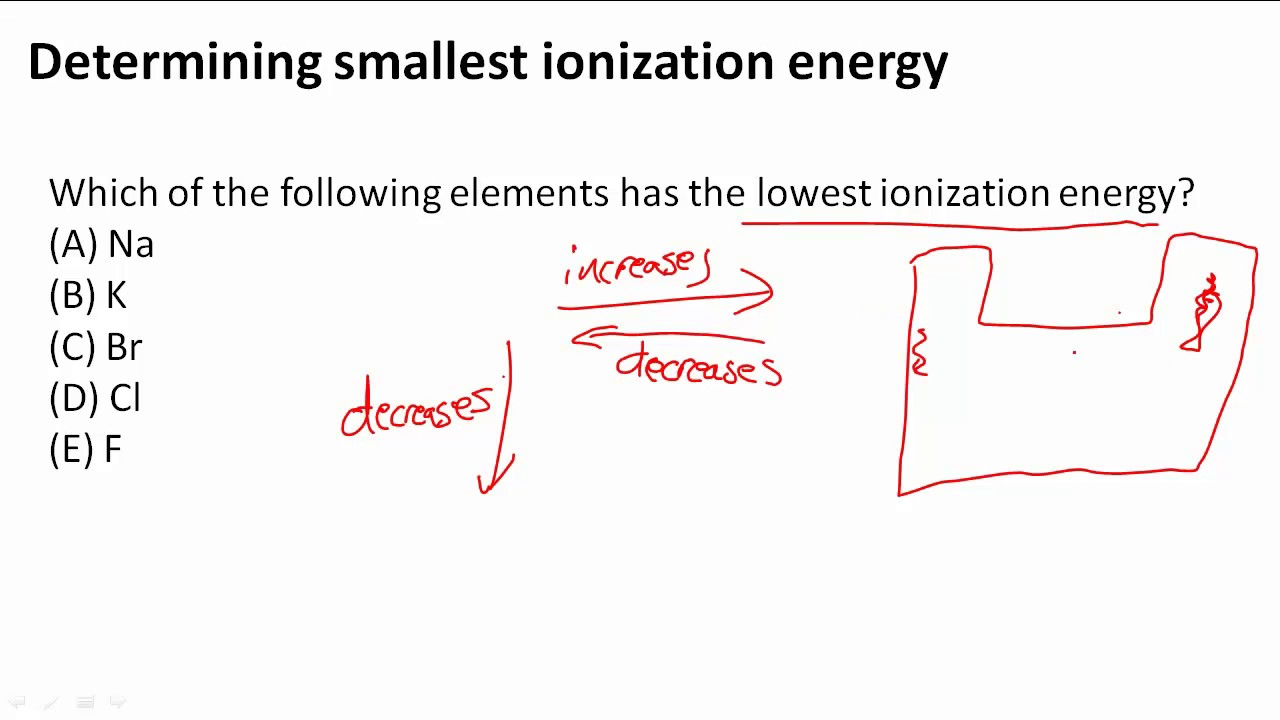
It is because of the shielding effect that the ionization energy decreases from top to bottom within a group. Which element has the lowest first ionization energy? The ionization energy decreases from top to bottom in groups, and increases from left to right across a period.
 Source: chegg.com
Source: chegg.com
Because after removing the two electron its electronic configuration have become 1s2,2s1. Among the following, which element has the lowest ionization energy? On moving down the group, the number of inner shells increase, the ionization energy decrease, the lowest ionisation energy is that of sulphur
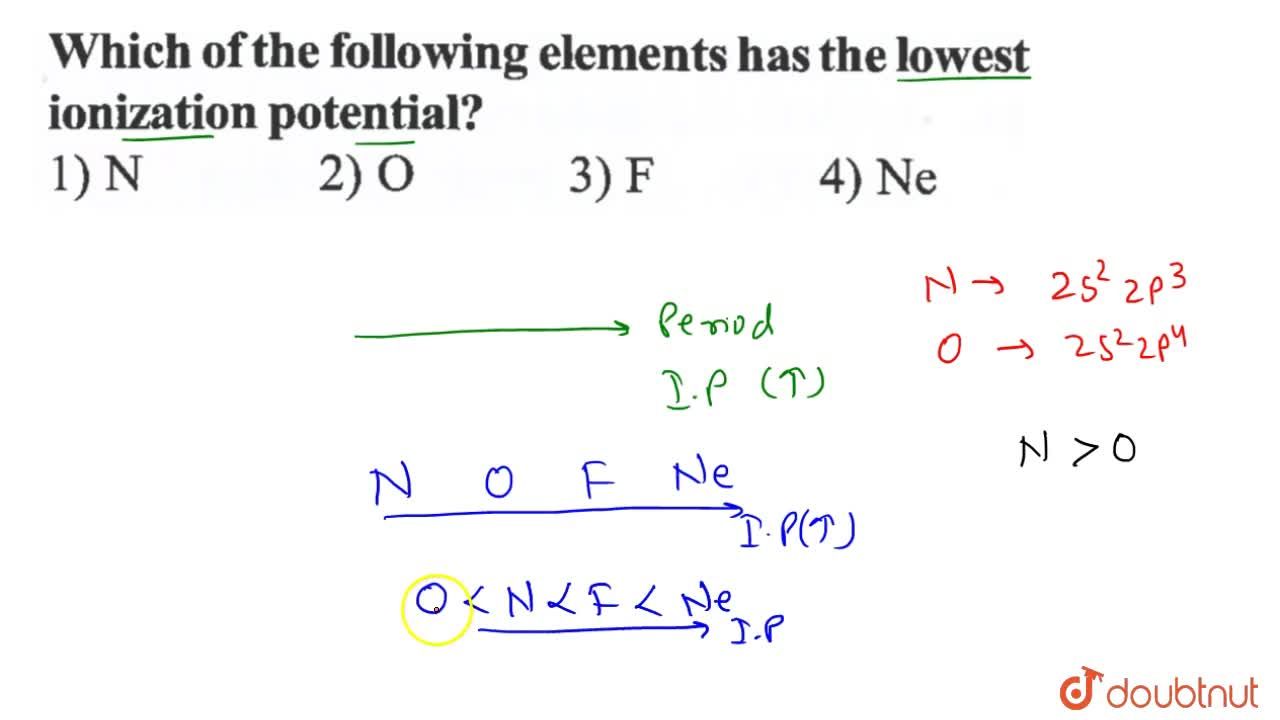 Source: doubtnut.com
Source: doubtnut.com
The ionization energy decreases from top to bottom in groups, and increases from left to right across a period. Of the following elements, which one has the lowest first ionization energy? 104 rows you can print the list of elements by hitting the print button below.
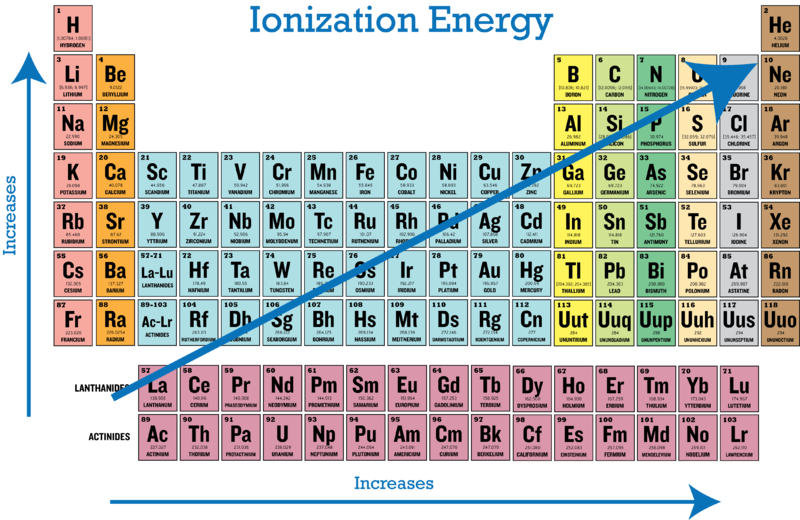
Thus, helium has the largest first ionization energy, while francium has one of the lowest. A period 3 element (1 point) ie1: Because after removing the two electron its electronic configuration have become 1s2,2s1.
 Source: youtube.com
Source: youtube.com
Group 1 of the periodic table features metals whose valence electron singly occupies the outermost shell, the valence shell of the given atoms. Group 1 of the periodic table features metals whose valence electron singly occupies the outermost shell, the valence shell of the given atoms. Is larger than the magnesium atom the shielding effect makes it easier to remove the outermost electrons form those atoms which have many electrons.
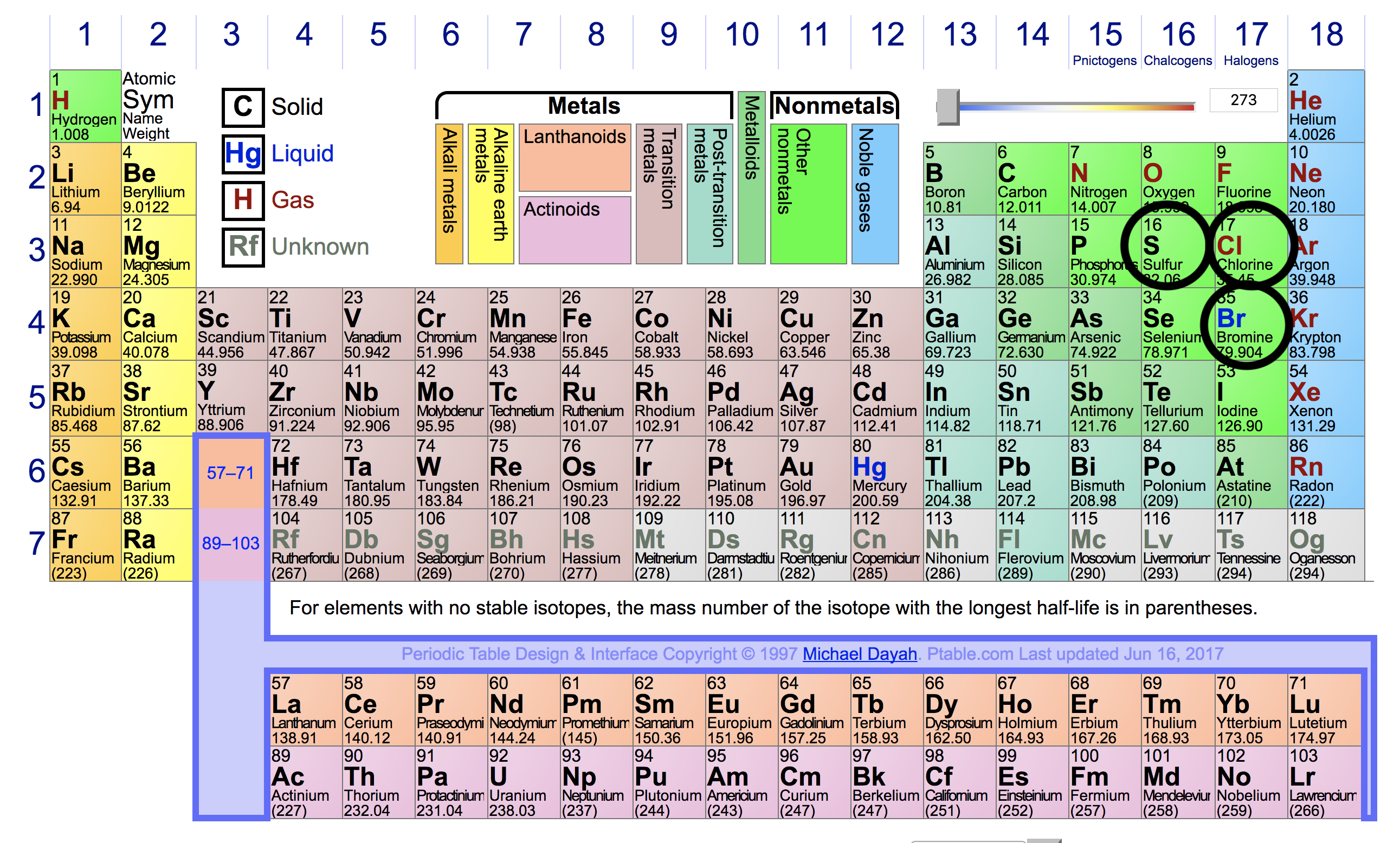 Source: socratic.org
Source: socratic.org
And the element which has the lowest ionization energy is caesium in 3.8939 ev. Down in a group ionization potential decreases so, the ionization potential of sulphur is lower than the oxygen. Now in the case of na and mg ,na has lower ionization energy and mg has higher.

I am not a gas at room temperature. 104 rows you can print the list of elements by hitting the print button below. Because after removing the two electron its electronic configuration have become 1s2,2s1.
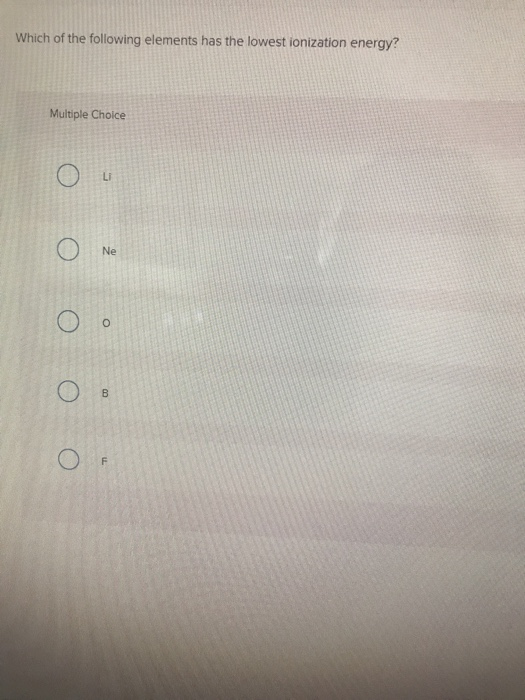 Source: chegg.com
Source: chegg.com
Why does boiling point decrease across a period? A period 3 element (1 point) ie1: Cesiumthe element with the lowest ionization energy is cesium (cs).

From this trend, cesium is said to have the lowest ionization energy and fluorine is said to have the highest ionization energy (with the exception of helium and neon). So, sulphur has the lowest ionization potential among all four. Is larger than the magnesium atom the shielding effect makes it easier to remove the outermost electrons form those atoms which have many electrons.
 Source: physicalsciencetext.weebly.com
Source: physicalsciencetext.weebly.com
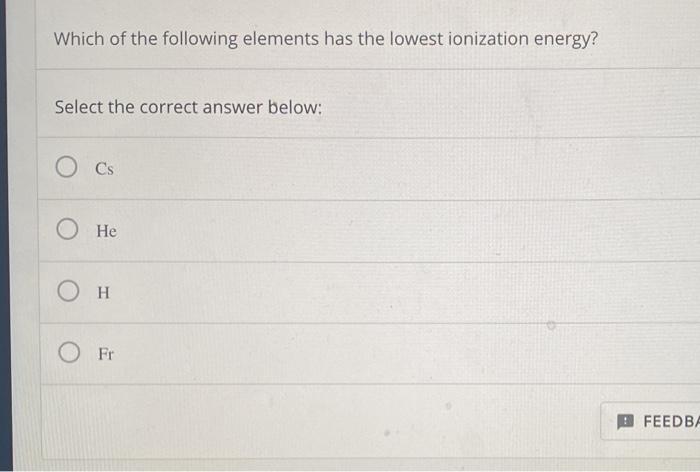
The element with the lowest ionization energy is cesium (cs). Which element has the lowest first ionization energy? Analyze each table of ionization energy data (in kj/mol) to identify each element.
Boron carbon aluminum silicon get the answers you need, now! Therefore, option (c) sulphur is correct. Ionization energy, also called ionization potential, in chemistry and physics, the amount of energy required to remove an.
 Source: angelo.edu
Source: angelo.edu
So, sulphur has the lowest ionization potential among all four. 104 rows you can print the list of elements by hitting the print button below. On moving down the group, the number of inner shells increase, the ionization energy decrease, the lowest ionisation energy is that of sulphur
 Source: clutchprep.com
Source: clutchprep.com
The first ionization energy varies in a predictable way across the periodic table. So,now arranging above mentioned atoms ( cl,p,ca) you�ll notice that calcium is in 2nd group at 3rd position,phosphorus lies in 5b group at 2nd position and chlorine lies in 7b group at 2nd position.so as calcium is below both atoms so it is larger in. An atom in the ground state contains three electrons in its outermost principal energy level.
Also Read :