Based on the indicated electronegativities, arrange the following in order of increasing ionic character: Which of the following elements has the lowest electronegativity?
Which Of The Following Elements Has The Lowest Electronegativity. The electronegativity of calcium (ca) is 1. In the following which configuration element has maximum electronegativity. A) 1 c) b) 6 d) 2 7. Based on the indicated electronegativities, arrange the following in order of increasing ionic character:

Related Post Harlan.k12.Ky.us :
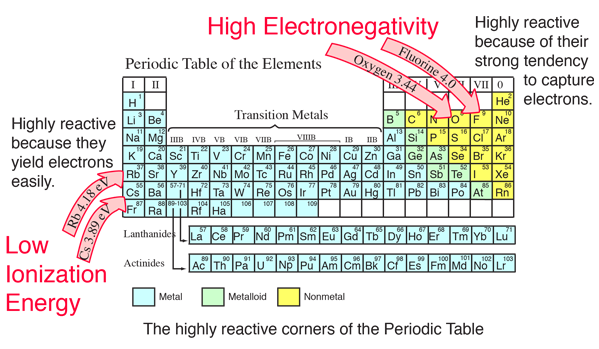
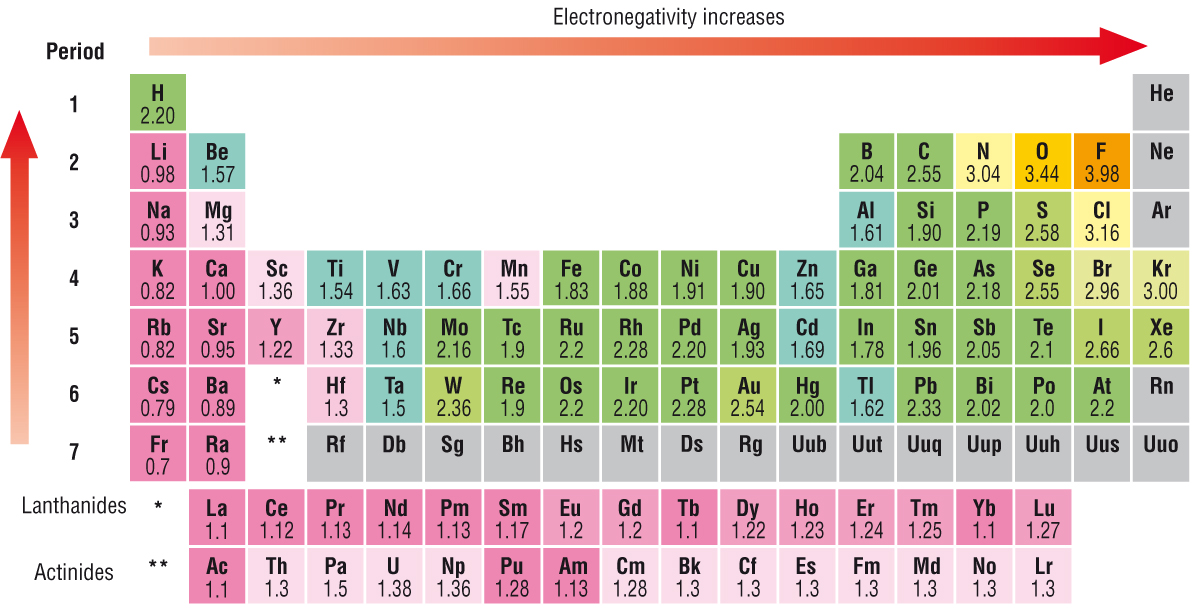
Element electronegativity br 2.8 p 2.1 mg 1.2 la 1.0 cs 0.7 Thus, fluorine is the most electronegative element, while francium is one of the least electronegative. As such, it has a lower tendency to attract electrons , and thus has a lower electronegativity. In the following which configuration element has maximum electronegativity.
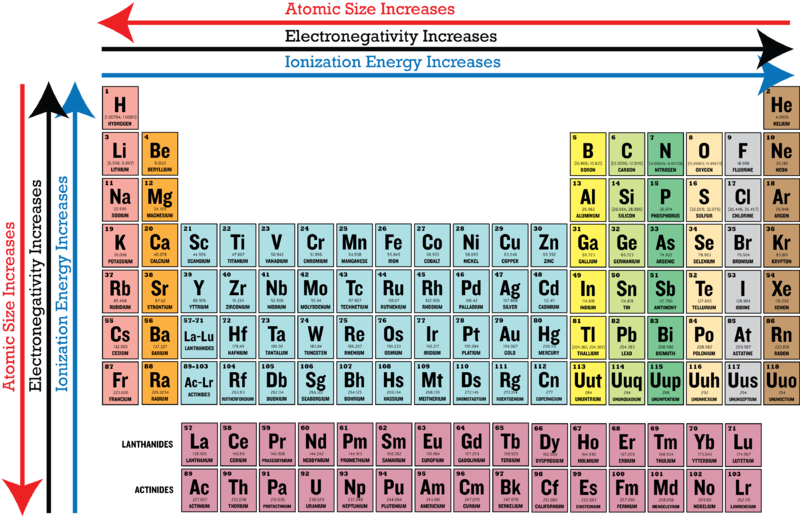
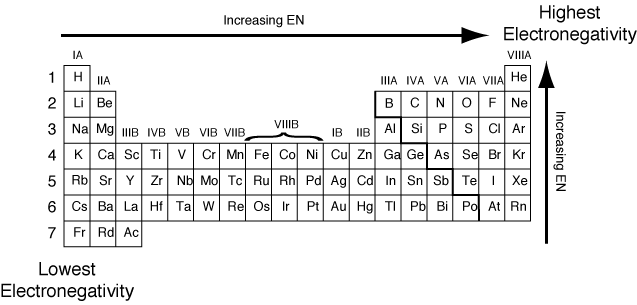
Electronegativity increases from bottom to top in groups, and increases from left to right across periods.
Which out of the following elements is the largest atom: Choice selenium (se) bromine (br) arsenic (as) germanium (ge) 10b) which of the following elements has the lowest electronegativity? Barium (ba) has the lowest electronegativity of the choices you have given. Which of the following elements has the lowest electronegativity? The allen scale assigns the lowest electronegativity to cesium, with a value of 0.659. Which of the following elements has the lowest electronegativity?
 Source: hyperphysics.phy-astr.gsu.edu
Source: hyperphysics.phy-astr.gsu.edu
Which of the following elements has the lowest electronegativity. And you must have known that when you go to the right the electronegativity increases so li is the lowest one as it is located at the left of this row. A) sodium c) aluminum b) potassium d) phosphorus.
 Source: slideplayer.com
Source: slideplayer.com
What 3 elements have the highest electronegativity? Which of the following elements has the lowest electronegativity? Barium (ba) has the lowest electronegativity of the choices you have given.

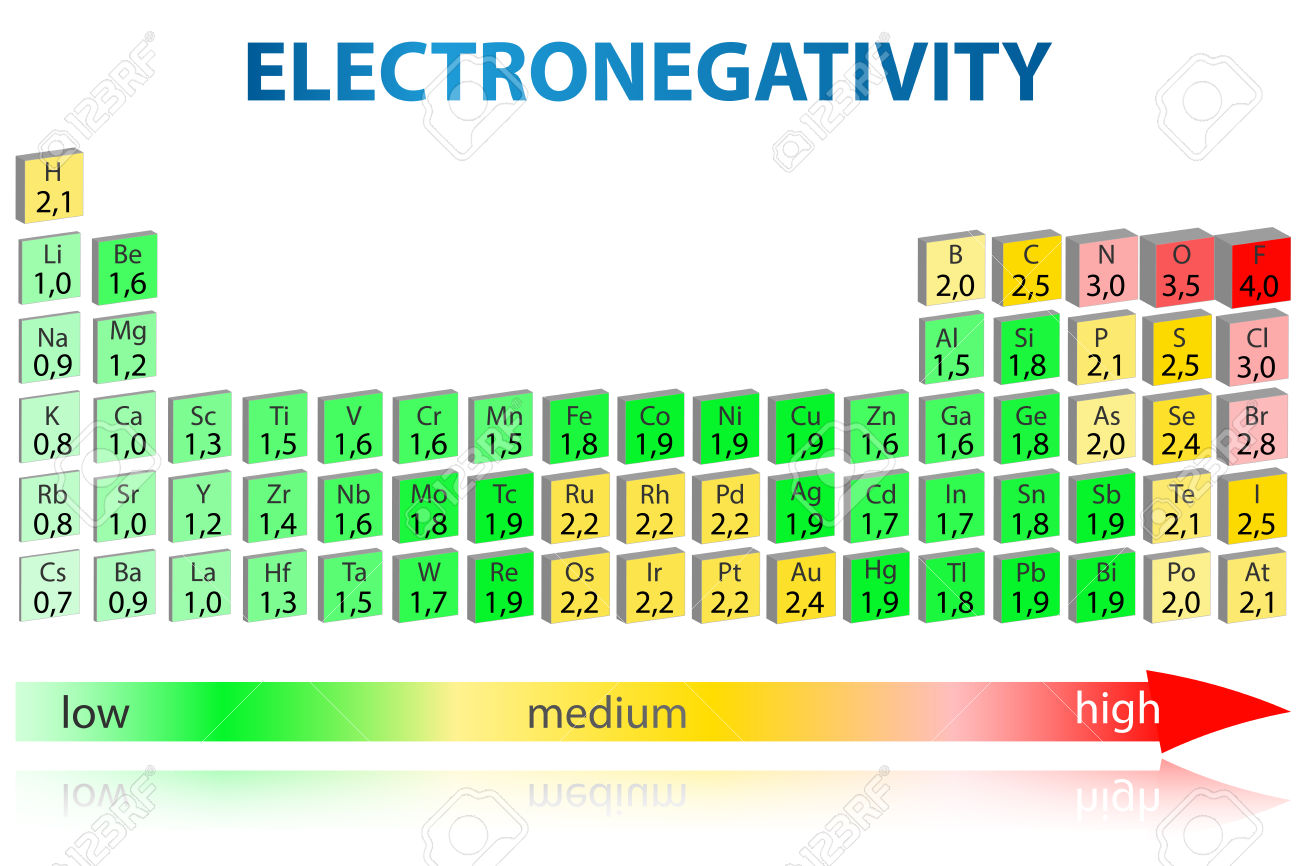
So, iodine has the lowest electronegativity. The electronegativity of magnseium (mg) is 1.31 (has the highest) the electronegativity of barium (ba) is 0.89. Based on the indicated electronegativities, arrange the following in order of increasing ionic character:
 Source: vedantu.com
Source: vedantu.com
Which out of the following elements is the largest atom: Hence, option d is correct. Choice selenium (se) bromine (br) arsenic (as) germanium (ge) 10b) which of the following elements has the lowest electronegativity?
 Source: quizlet.com
Source: quizlet.com
Lithium, beryllium, magnesium, sodium 2. Barium (ba) has the lowest electronegativity of the choices you have given. Which of the following elements has the lowest electronegativity?
 Source: socratic.org
Source: socratic.org
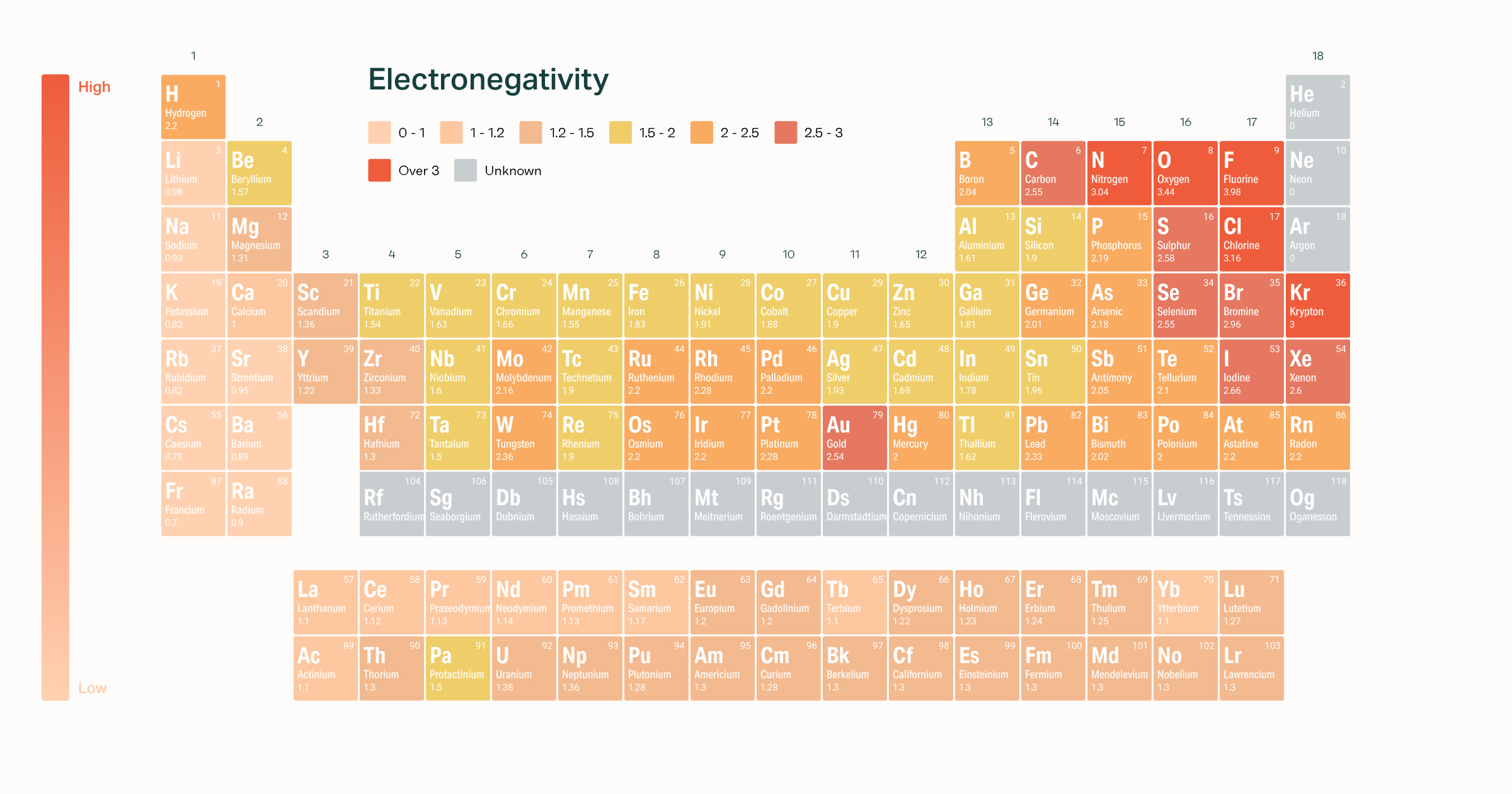
Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. C nitrogen has a greater number of electrons in a similar volume, resulting in greater electronegativity.; The electronegativity of magnseium (mg) is 1.31 (has the highest) the electronegativity of barium (ba) is 0.89.
 Source: slideplayer.com
Source: slideplayer.com
Of the list above, you can see that barium has the lower level of electronegativity (0.89). Therefore, the correct answer is d. Fluorine has the highest electronegativity followed by chlorine, bromine and then with the least reactivity we have iodine,amongst the given options.
 Source: breakingatom.com
Source: breakingatom.com
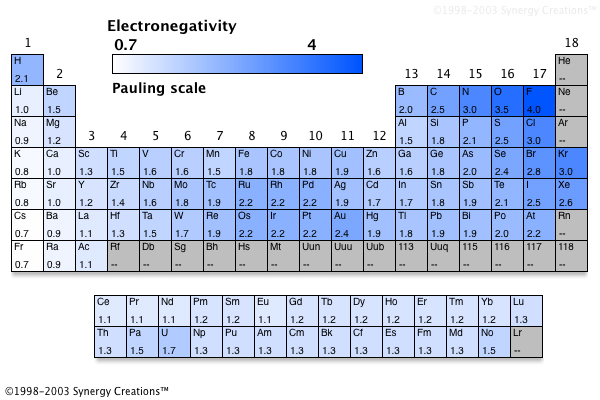
Fluorine (the most electronegative element) is assigned a value of 4.0, and values range down to caesium and francium which are the least electronegative at 0.7. The element with the lowest electronegativity value is francium which has an electronegativity of 07. A) sodium c) aluminum b) potassium d) phosphorus.
 Source: toppr.com
Source: toppr.com
Electronegativity increases from bottom to top in groups, and increases from left to right across periods. Thus, fluorine is the most electronegative element, while francium is one of the least electronegative. A measure of the ability of an atom in a chemical compound to attract electrons from another atom in the compound is called.
 Source: sciencenotes.org
Source: sciencenotes.org
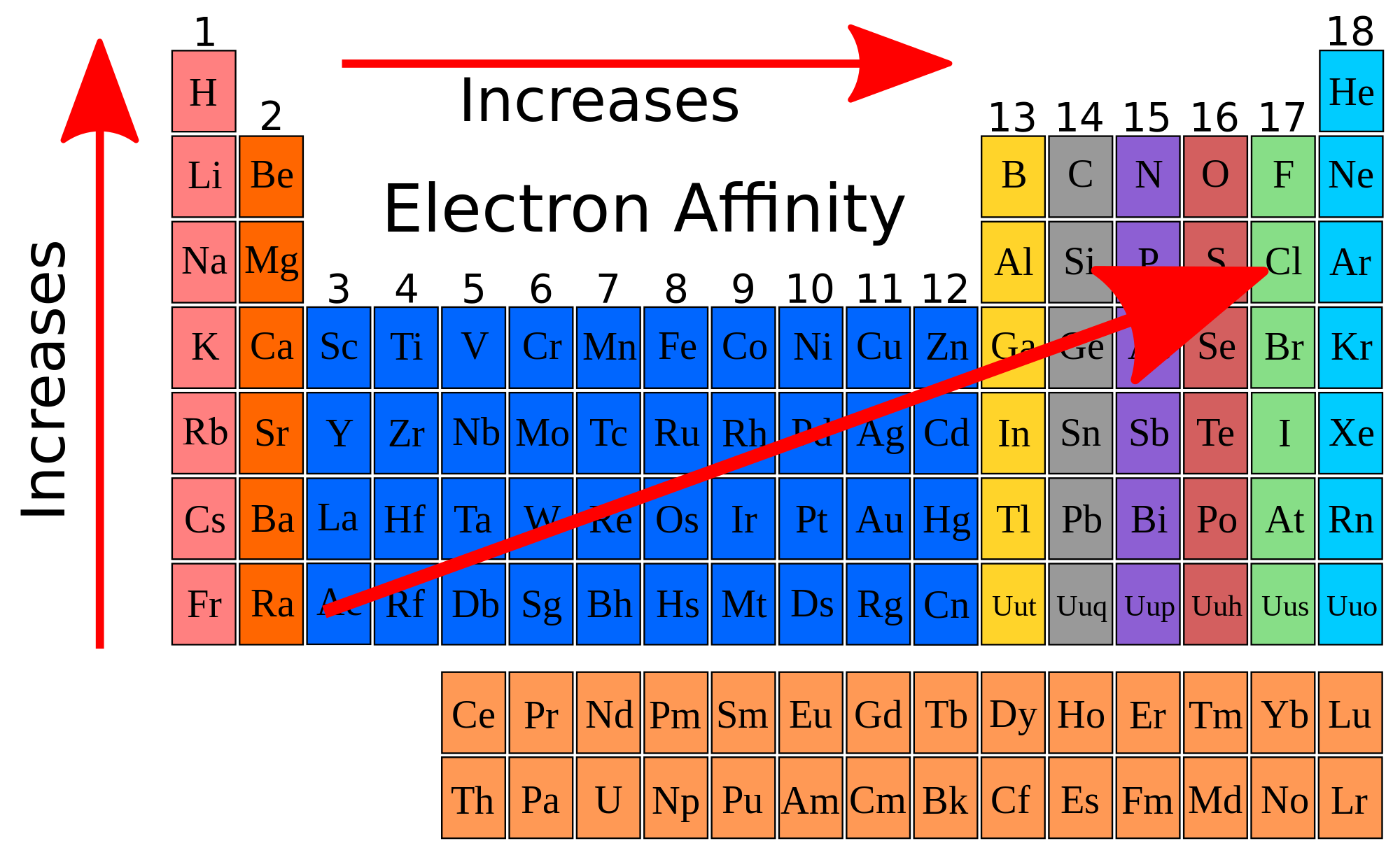
What are the name and group number of the chemical family that has the lowest overall electronegativities? In the case of carbon, bromine and fluorine, they all can gain electrons to achieve full outer shells (fluorine is in fact the most electronegative atom). The lowest electronegativity of the element from the following atomic number is.
 Source: socratic.org
Source: socratic.org
Atoms with a low electronegativity, like lithium, have a weak attractive force for electrons because? Group 18 elements have no electronegativity. The element with the lowest electronegativity value is francium which has an electronegativity of 07.
 Source: thoughtco.com
Source: thoughtco.com
It has the atomic number of 55. The alkaline earth element having the largest atomic radius is found in period: Choice calcium (ca) nitrogen (n) silicon (si) rubidium (rb) 10 10a) which of the following elements has the highest electronegativity?
 Source: socratic.org
Source: socratic.org
The electronegativity of magnseium (mg) is 1.31 (has the highest) the electronegativity of barium (ba) is 0.89. In the case of carbon, bromine and fluorine, they all can gain electrons to achieve full outer shells (fluorine is in fact the most electronegative atom). Was asked on may 31 2017.
 Source: socratic.org
Source: socratic.org
This value uses the pauling scale to measure electronegativity. One of the least electronegative elements would be found on the periodic table in. Which of the following elements has the lowest electronegativity.
 Source: angelo.edu
Source: angelo.edu
A nitrogen has a greater number of protons and hence an effective nuclear charge that more strongly attracts the bonding electrons.; In the case of carbon, bromine and fluorine, they all can gain electrons to achieve full outer shells (fluorine is in fact the most electronegative atom). The alkali metals as a group have the lowest electronegativities, with the values falling as the atomic number increases, so the winner is caesium (cesium), with a pauling score of 0.79.
 Source: socratic.org
Source: socratic.org
Hence, option d is correct. 119 rows element electronegativity: Which element has the lowest electronegativity?

A nitrogen has a greater number of protons and hence an effective nuclear charge that more strongly attracts the bonding electrons.; So such an element is fluorine in the given option whose electronegativity is 4.0. Electronegativity increases from bottom to top in groups, and increases from left to right across periods.
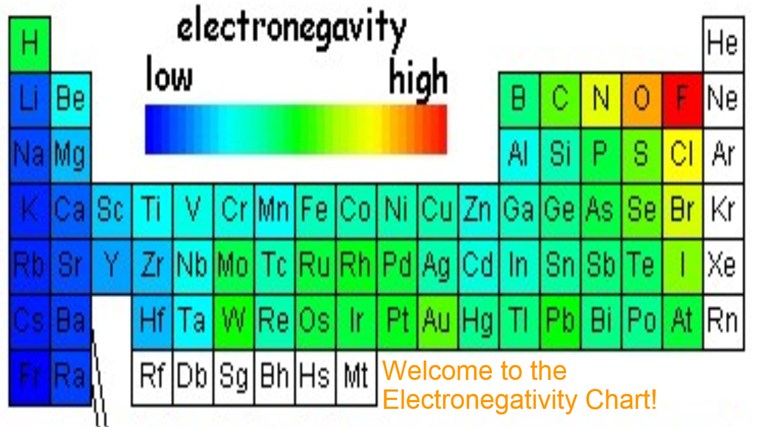 Source: periodictable.me
Source: periodictable.me
Atoms with a low electronegativity, like lithium, have a weak attractive force for electrons because? So such an element is fluorine in the given option whose electronegativity is 4.0. Fluorine has the highest electronegativity followed by chlorine, bromine and then with the least reactivity we have iodine,amongst the given options.
Electronegativity increases from bottom to top in groups, and increases from left to right across periods. Lithium, beryllium, magnesium, sodium 3. Francium has the lowest electronegativity value (0.7).
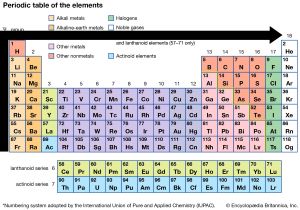 Source: study.com
Source: study.com
Francium has the lowest electronegativity value (0.7). Which of the following elements has the highest electronegativity? The alkaline earth element having the largest atomic radius is found in period:
Also Read :





