Covalent bonds can be occurred between any two atoms. B) only covalent bonds can form between molecules.
Which Of The Following Distinguishes Hydrogen Bonds From Covalent Bonds. The two strands of the double helix would separate b. Therefore, it will form covalent bonds with three hydrogen atoms. What would first happen to dna molecules treated with dnaase? Which of the following is not a result of hydrogen bonds?
 Bonds In Biology Weak Bonds Strong Bonds Hydrogen Bond Hydrogen Bonds - Ppt Download From slideplayer.com
Bonds In Biology Weak Bonds Strong Bonds Hydrogen Bond Hydrogen Bonds - Ppt Download From slideplayer.com
Related Post Bonds In Biology Weak Bonds Strong Bonds Hydrogen Bond Hydrogen Bonds - Ppt Download :
Only hydrogen bonds can form between molecules. A)ionic bonds form between the same two elements, whereas covalent bonds form between different elements. Which of the following is not a result of hydrogen bonds? Covalent bonds (also known as homopolar bonds).
What distinguishes hydrogen bonds from covalent bonds?
A)ionic bonds form between the same two elements, whereas covalent bonds form between different elements. The covalent bonds are intramolecular bonds because they hold the atoms together in a single molecule. Covalent bonds form when two atoms share electrons. • covalent bonds result between atoms to produce a molecule. In this case the electronegativity difference is low. • covalent bonds are stronger than hydrogen bonds.
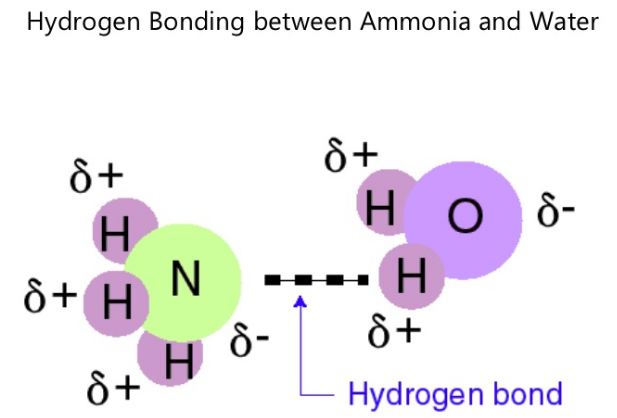 Source: vivadifferences.com
Source: vivadifferences.com
Covalent, ionic, hydrogen which of the following distinguishes hydrogen bonds from covalent bonds? A single covalent bond between two atoms is due to an electron pair shared by the atoms. Only hydrogen bonds can form between molecules.
 Source: slideplayer.com
Source: slideplayer.com
Two hydrogen atoms join together to form a molecule of hydrogen gas. Generally the atoms of the same element are involved in covalent bonding. D) only hydrogen bonds can.
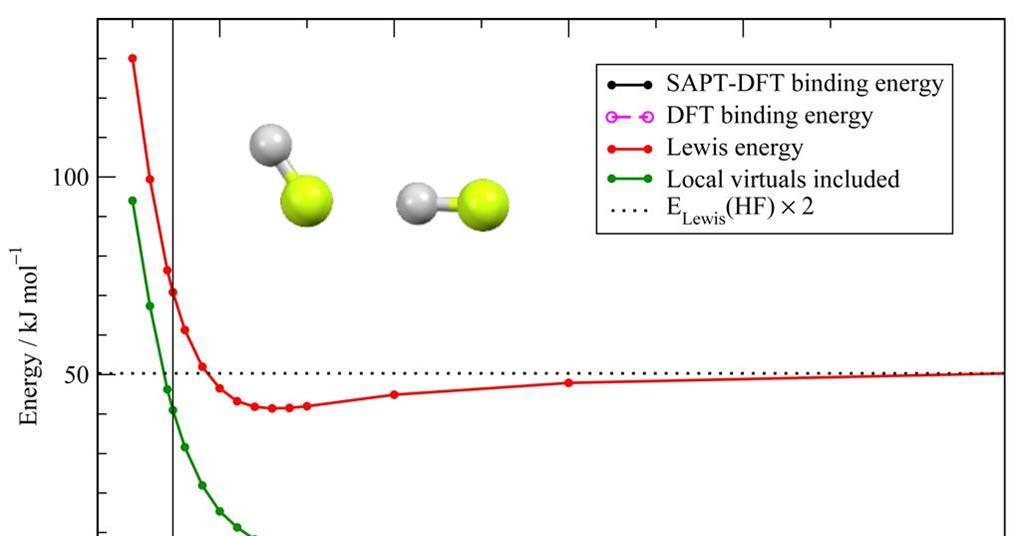 Source: chemistryworld.com
Source: chemistryworld.com
Covalent bond strengths range from 100 kj/mol to 1100 kj/mol. Each single covalent bond includes the sharing of three pairs of electrons. They occur between, for instance, hydrogen, carbon, nitrogen, and oxygen.
 Source: socratic.org
Source: socratic.org
B.) only hydrogen bonds can form within molecules. Which of the following distinguishes hydrogen bonds from covalent bonds? B.) only hydrogen bonds can form within molecules.
 Source: coursehero.com
Source: coursehero.com
Hydrogen bonds are not exactly bonds, but they can be better considered as forces of attraction. Hydrogen bonding is strongest between molecules of 1. Covalent bonds are stronger than disulfide bonds.
 Source: coursehero.com
Source: coursehero.com
Covalent bonds are stronger than disulfide bonds. Only hydrogen bonds can form between molecules. Covalent bonds are less elastic than hydrogen bonds.
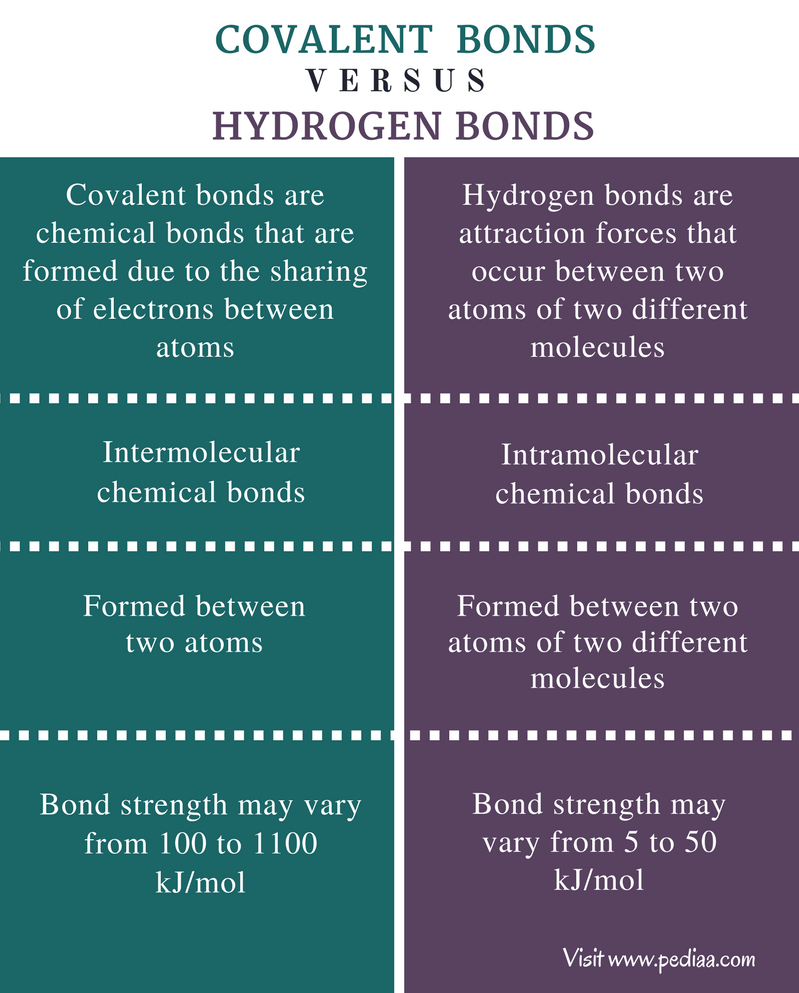 Source: pediaa.com
Source: pediaa.com
A single covalent bond between two atoms is due to an electron pair shared by the atoms. Hydrogen bonds are not exactly bonds, but they can be better considered as forces of attraction. C.) only hydrogen bonds can form between molecules.
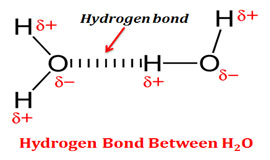 Source: easybiologyclass.com
Source: easybiologyclass.com
Which of the following distinguishes ionic bonds from covalent bonds? A)ionic bonds form between the same two elements, whereas covalent bonds form between different elements. Therefore, it will form covalent bonds with three hydrogen atoms.

• hydrogen atom should be there to have a hydrogen bond. D) only hydrogen bonds can. Traditionally one distinguishes the following types of bonds:
 Source: onlychemistrydiscussion.blogspot.com
Source: onlychemistrydiscussion.blogspot.com
Generally the atoms of the same element are involved in covalent bonding. The purines would be separated from the deoxyribose sugar d. D) only hydrogen bonds can.
 Source: coursehero.com
Source: coursehero.com
Ionic bonds form between the same two elements, whereas covalent bonds form between different elements. Only covalent bonds can form between molecules. Covalent bonds are stronger than hydrogen bonds.
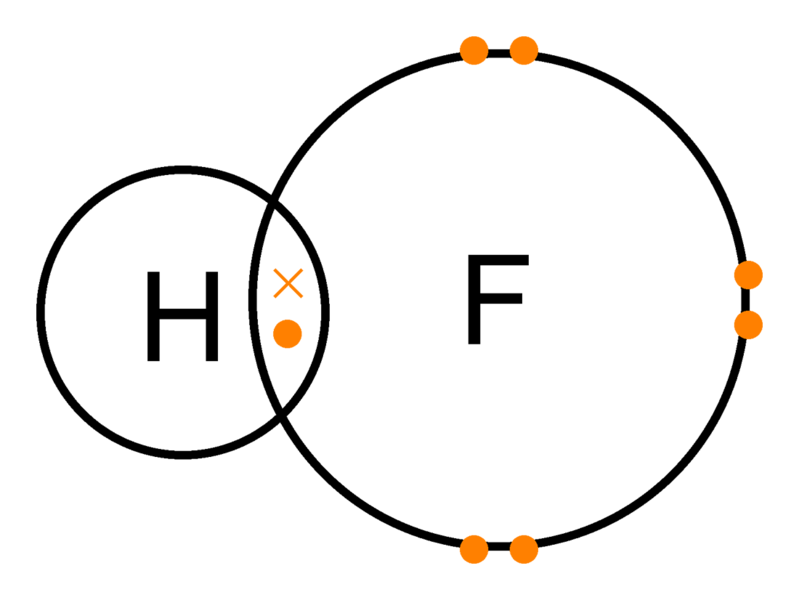
 Source: easybiologyclass.com
Source: easybiologyclass.com
Covalent bonds can be occurred between any two atoms. What distinguishes hydrogen bonds from covalent bonds? Only hydrogen bonds can form between molecules.
![Chemical Bonds | Microbiology [Master] Chemical Bonds | Microbiology [Master]](https://s3-us-west-2.amazonaws.com/courses-images/wp-content/uploads/sites/1950/2017/05/31183005/figure-02-01-11.jpeg) Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
Individual hydrogen bonds are weak bonds however, their presence in large number provide them considerable strength. Which of the following is not a result of hydrogen bonds? Covalent bonds are stronger than hydrogen bonds.
 Source: youtube.com
Source: youtube.com
In this case the electronegativity difference is low. The phosphodiester bonds between deoxyribose sugars would be broken. Which of the following distinguishes hydrogen bonds from covalent bonds?
 Source: britannica.com
Source: britannica.com
Which of the following is not a result of hydrogen bonds? Traditionally one distinguishes the following types of bonds: This results in the separation of sodium from chloride, thus breaking the ionic bond.
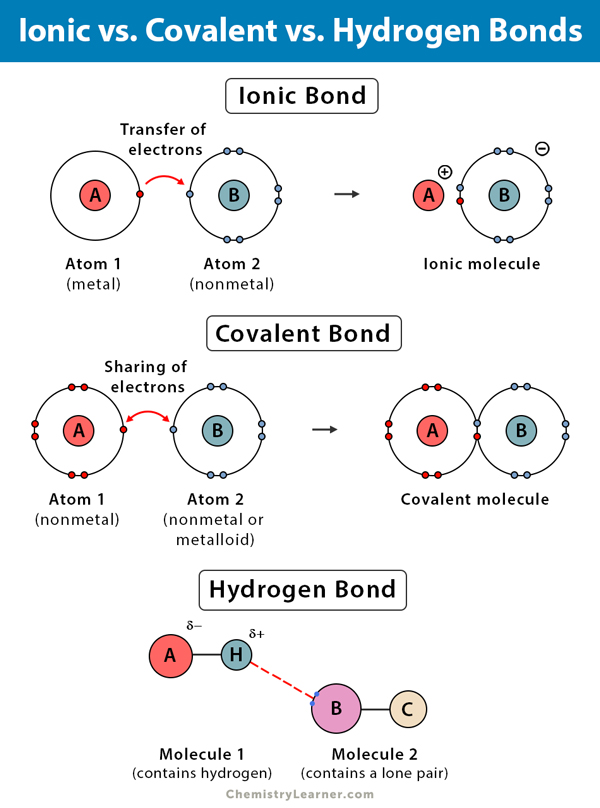
 Source: pediaa.com
Source: pediaa.com
Only hydrogen bonds can form between molecules. Which factor distinguishes a metallic bond from an ionic bond or a covalent bond how do electronegativity values determine the charge distribution in a polar covalent bond the sun produces energy by fusing hydrogen atoms into ____ atoms in its core. Which of the following is not a result of hydrogen bonds?
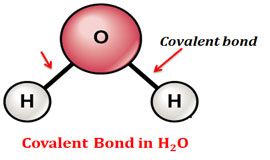
 Source: chemistrylearner.com
Source: chemistrylearner.com
Which of the following distinguishes hydrogen bonds from covalent bonds? Nitrogen has 5 electrons in its valence (outermost) electron shell. Which of the following distinguishes hydrogen bonds from covalent bonds?
 Source: sciencedirect.com
Source: sciencedirect.com
Only covalent bonds can form between molecules. Metallic bonds are the chemical bonds that join metals to metals. The covalent bond is also called a shared bond.
 Source: sciencedirect.com
Source: sciencedirect.com
Only hydrogen bonds can form between molecules. Which of the following distinguishes hydrogen bonds from covalent bonds? Which of the following distinguishes hydrogen bonds from covalent bonds?
 Source: pediaa.com
Source: pediaa.com
Which of the following distinguishes hydrogen bonds from covalent bonds? The covalent bond is also called a shared bond. Covalent bonds are stronger than disulfide bonds.
Also Read :





