Which of the following compounds is a strong arrhenius base? Arrhenius base are defined as the specie which when dissolved in water dissociate to give hydroxide ions.
Which Of The Following Compounds Is An Arrhenius Base. Which of the following is an arrhenius base? Recall that according to the arrhenius definition: According to him, an acid is a compound which can readily give up protons or hydrogen ion in aqueous or water solution. The reaction between an arrhenius acid and an arrhenius base is called neutralization and results in the formation of water and a salt.

Related Post Solved Which Of The Following Is An Arrhenius Base? Ch3Co2H | Chegg.com :
More than one of these compounds is an arrhenius base. Acid is a compound that dissolves in water releasing h + ions. Classify each of these compounds as an arrhenius acid, an arrhenius base, or. The example for arrhenius base is highly soluble sodium hydroxide compound in water, which dissociates to give sodium ion and hydroxide ion.
The reaction between an arrhenius acid and an arrhenius base is called neutralization and results in the formation of water and a salt.
An arrhenius base is a compound that increases the oh − ion concentration in aqueous solution. According to arrhenius, the definitions of acids and bases, namely: When dissolved in water, which of the following compounds is an arrhenius acid? When dissolved in water, which of the following compounds is an arrhenius base? An arrhenius acid is a compound that increases the h + ion concentration in aqueous solution. C) a strong arrhenius base.

Ch3co2h koh ch3oh nabr more than one of these compounds is an arrhenius base. I call so2 an arrhenius acid, also, because it reacts with water to form. Acid is a compound that dissolves in water releasing h + ions.
 Source: clutchprep.com
Source: clutchprep.com
An arrhenius base is a compound that increases the oh − ion concentration in aqueous solution. A base is a compound which, when dissolved in water, releases oh− ions. The example for arrhenius base is highly soluble sodium hydroxide compound in water, which dissociates to give sodium ion and hydroxide ion.
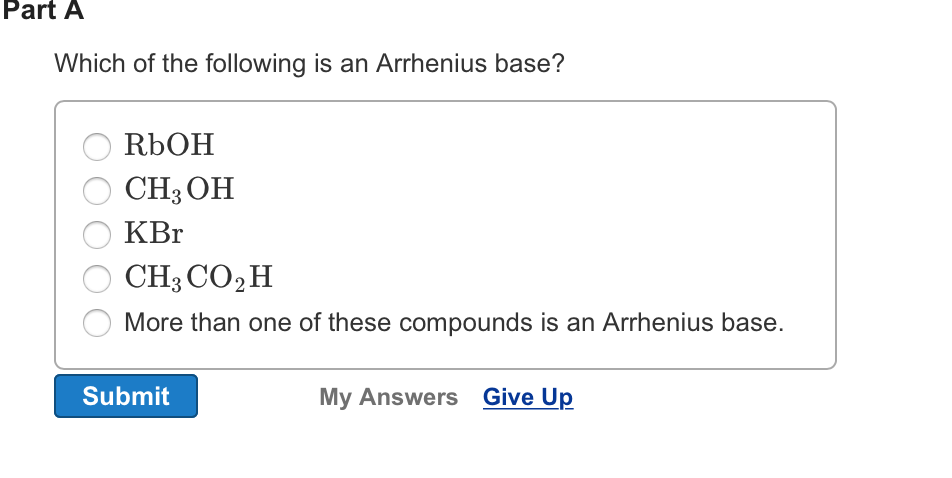 Source: chegg.com
Source: chegg.com
An arrhenius base is a compound that increases the oh − ion concentration in aqueous solution. Which of the following is an arrhenius base?a. Hydrochloric acid (hcl) which is very soluble in water is classified as arrhenius acid, as hcl can
 Source: chegg.com
Source: chegg.com
Which of the following is an arrhenius base? Which of the following is an arrhenius base? C6h12o6 h2so4 c 3 h 7 nh 2 hocl 2.
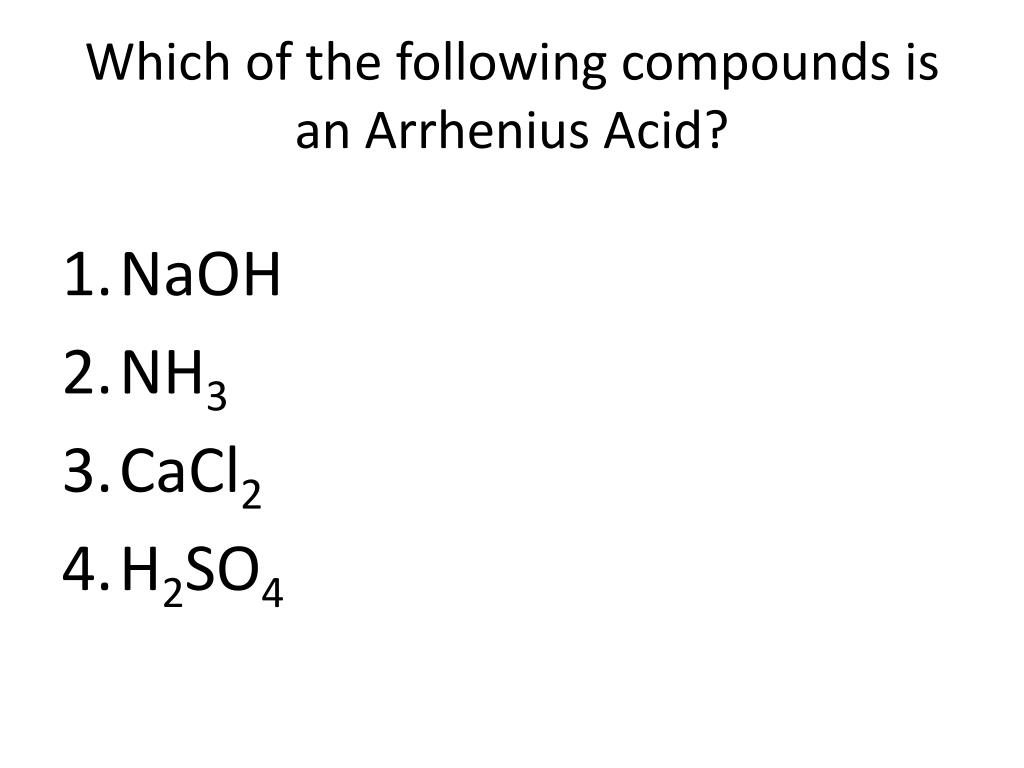 Source: slideserve.com
Source: slideserve.com
Arrhenius base are defined as the specie which when dissolved in water dissociate to give hydroxide ions. Acid is a compound that dissolves in water releasing h + ions. In aqueous solution, ions immediately react with water molecules to form hydronium ions,.

An acid is a substance that produces h^+ when in water solution and more modern forms suggest replacing h^+ with h3o^+ (hydrogen ion with hydronium ion). When dissolved in water, which of the following compounds is an arrhenius acid? E) a weak arrhenius acid.

According to arrhenius, the definitions of acids and bases, namely: A net ionic equation for the neutralization reaction of hcn (aq) with naoh (aq). Classify each of these compounds as an arrhenius acid, an arrhenius base, or neither.hclo3 kclnaoh hg(oh) 2h3po4 al(oh) 3hcl hbrzn(oh)2 c 3h8.
 Source: clutchprep.com
Source: clutchprep.com
The substance h2so3 is considered a) a weak arrhenius base. An arrhenius base is any species that increases the concentration of in aqueous solution. When dissolved in water, which of the following compounds is an arrhenius base?
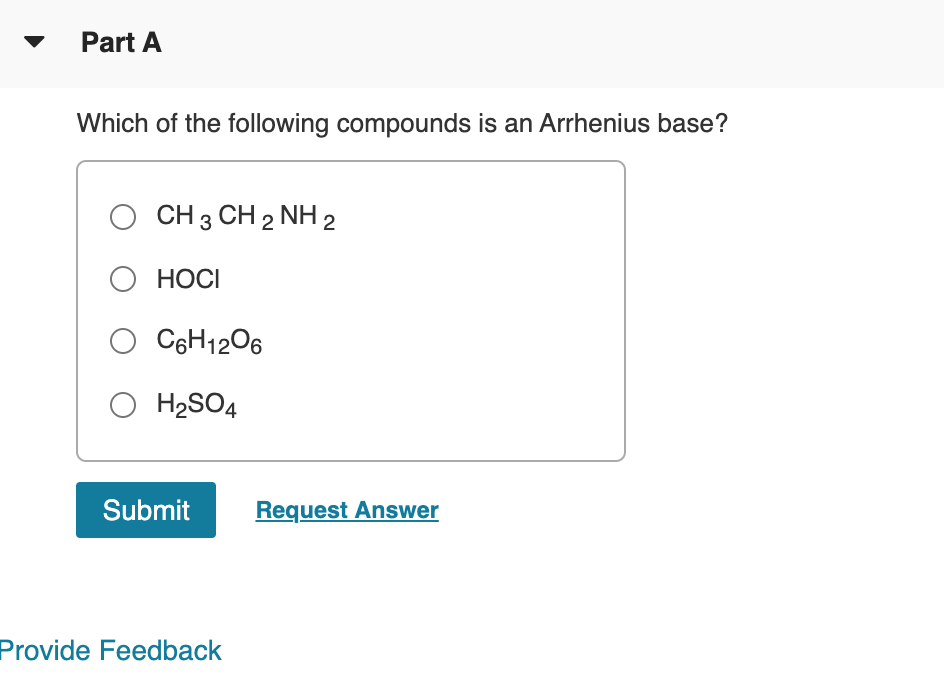 Source: chegg.com
Source: chegg.com
According to arrhenius, the definitions of acids and bases, namely: The reaction between an arrhenius acid and an arrhenius base is called neutralization and results in the formation of water and a salt. An arrhenius base is any species that increases the concentration of in aqueous solution.
 Source: chegg.com
Source: chegg.com
Typical arrhenius acids include the common mineral acids such as hydrochloric acid, sulphuric acid, etc. A) ch3co2h b) koh c) ch3oh d) licl e) more than one of these compounds is an arrhenius base. Which of the following is an arrhenius base?
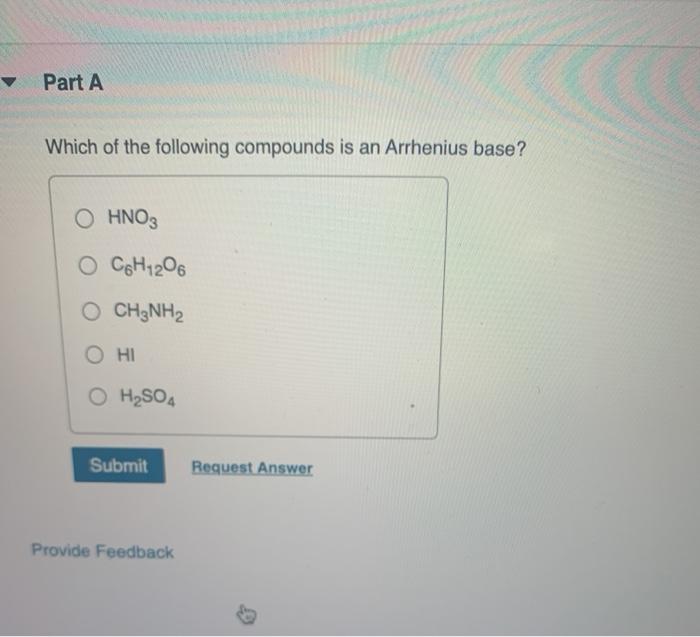
Which of the following compounds is a strong arrhenius base? Which of the following is an arrhenius base?a. When dissolved in water, which of the following compounds is an arrhenius base?
 Source: chegg.com
Source: chegg.com
Classify each of these compounds as an arrhenius acid, an arrhenius base, or. Which of the following is an arrhenius base? 43) which one of the following compounds behaves as an acid when dissolved in water?
 Source: chegg.com
Source: chegg.com
A net ionic equation for the neutralization reaction of hcn (aq) with naoh (aq). Classify each of these compounds as an arrhenius acid, an arrhenius base, or. Which of the following is an arrhenius base?a.

We’re being asked to determine which of the following given compounds is an arrhenius base. Ch3co2h koh ch3oh nabr more than one of these compounds is an arrhenius base. In aqueous solution, ions immediately react with water molecules to form hydronium ions,.

When dissolved in water, which of the following compounds is an arrhenius base? B) a strong arrhenius acid. A) ch_3 co_2 h b) naoh c) ch_3 oh d) licl e) more than.

An arrhenius acid is a compound that increases the h + ion concentration in aqueous solution. A) ch_3 co_2 h b) naoh c) ch_3 oh d) licl e) more than. Typical arrhenius acids include the common mineral acids such as hydrochloric acid, sulphuric acid, etc.

The example for arrhenius base is highly soluble sodium hydroxide compound in water, which dissociates to give sodium ion and hydroxide ion. For example, being an ionic compound when dissolved in water will give and ions. When dissolved in water, which of the following compounds is an arrhenius base?
 Source: chegg.com
Source: chegg.com
Recall that according to the arrhenius definition: A swedish scientist svante arrhenius, in the year 1884, proposed acid and base as the two classifications of compounds. Which of the following is an arrhenius base?
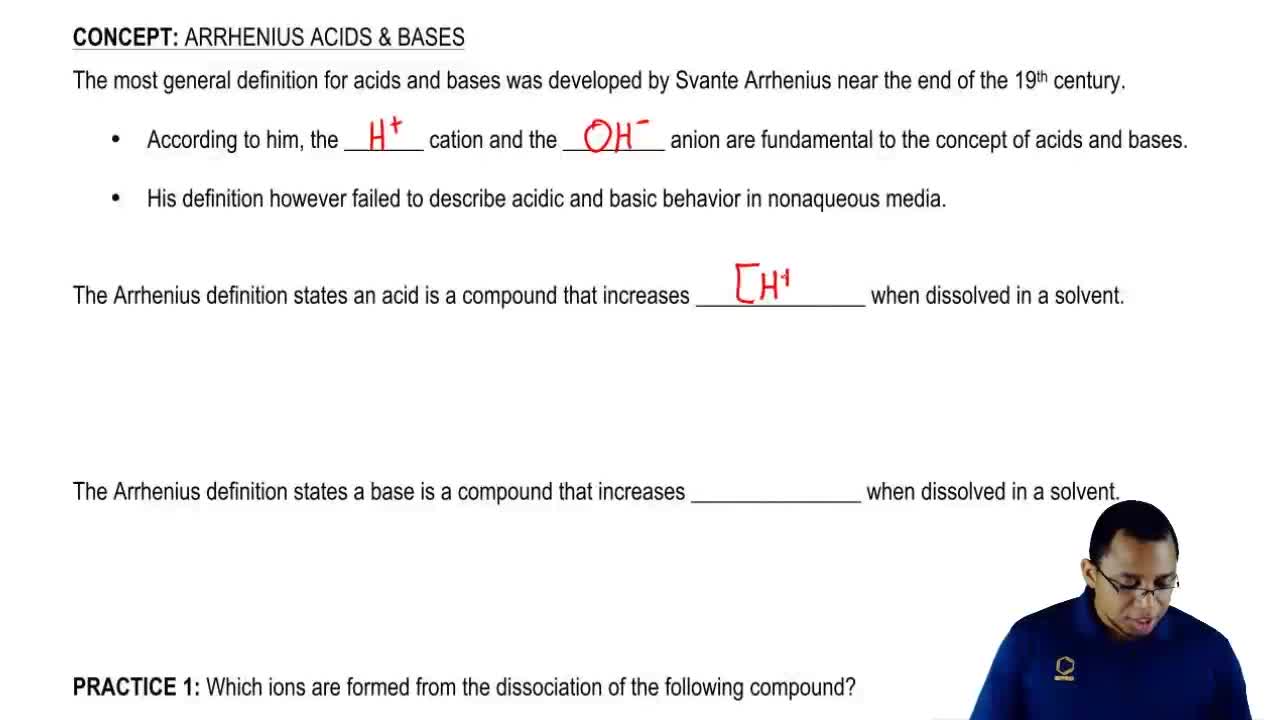 Source: clutchprep.com
Source: clutchprep.com
- which one of the following compounds behaves as an acid when dissolved in water? Arrhenius base are defined as the specie which when dissolved in water dissociate to give hydroxide ions. To recognize the arrhenius base look for a molecule ending in oh, but not following chx which refers to an alcohol.
 Source: clutchprep.com
Source: clutchprep.com
A net ionic equation for the neutralization reaction of hcn (aq) with naoh (aq). Hydrochloric acid (hcl) which is very soluble in water is classified as arrhenius acid, as hcl can An arrhenius base is a compound that increases the oh − ion concentration in aqueous solution.
Also Read :





