Sodium carbonate () is soluble in water. Asked aug 7, 2021 in nursing by pockypokey.
Which Of The Following Compounds Are Soluble In Water. Ethylene glycol has two hydroxy groups both of which form hydrogen bonds with water. Lead chloride is also insoluble in cold water but is soluble in hot water. According to rule three, the compound formed by c l minus and pb. Silver nitrate, or agn o3, is soluble because all nitrates are soluble, without exception.
 Answered: Which Of The Following Compounds Is… | Bartleby From bartleby.com
Answered: Which Of The Following Compounds Is… | Bartleby From bartleby.com
Related Post Answered: Which Of The Following Compounds Is… | Bartleby :
A white solid is observed to be insoluble in water, insoluble in excess ammonia. There are several major types of compounds that are soluble in water. The chemicals have to be exposed to their boiling point to fully dissolve. Tap card to see definition 👆.
Rank the following compounds in order of their solubility in water.
Which compounds are soluble in water. B a c l 2 and (n h 4 ) 2 c 2 o 4 are soluble in water as these can be dissociated into constituents ions in water. The halide salts of silver and lead are also insoluble in water. There are several major types of compounds that are soluble in water. Almost all ionic compounds are soluble in water but there are few exceptions. A polar molecule is one that�s neutral, or uncharged, but has an asymmetric internal distribution of.
 Source: transtutors.com
Source: transtutors.com
How do you know if a salt is soluble? So, we can say c 6 h 5 n o 2 is soluble in benzene but almost insoluble in water. A polar molecule is one that�s neutral, or uncharged, but has an asymmetric internal distribution of.
 Source: clutchprep.com
Source: clutchprep.com
For more detailed information of the exact solubility of the compounds, see the solubility table. Which compounds are soluble in water. Lipid solubility which of the following compounds would you expect to be most soluble in water?
 Source: numerade.com
Source: numerade.com
Click again to see term 👆. Almost all ionic compounds are soluble in water but there are few exceptions. Option (b) is the correct answer.
 Source: chegg.com
Source: chegg.com
The lattric enthalpies of agbr and agl are even higher because of greater number of electrons in their anions. (iii) formic acid is water soluble. The lattice enthalpy of agf is the lowest because of the least number of electon in its anion.

So we need to reference our scalability rules. Consequently, they are les soluble than agci. For more detailed information of the exact solubility of the compounds, see the solubility table.
 Source: transtutors.com
Source: transtutors.com
Thus, agf is water soluble. D) fecl3 eco, 7) if you had an aqueous mixture that contained as, k, and pb * cations, how many diff solids could precipitate if a chloride solution was added? Sodium chloride (nacl) table salt, or sodium chloride (nacl) , the most common ionic compound, is soluble in water (360 g/l).
 Source: clutchprep.com
Source: clutchprep.com
Which compound is soluble in cold water? (iii) formic acid is water soluble. Predict whether the following compounds are soluble or insoluble in water:
 Source: chegg.com
Source: chegg.com
(ii) toluene is water insoluble. The solubility rules tell you that all halides are soluble with the exception of those formed with siver, mercury, and lead ions. In a base, dichromates convert into chromates and some of the chromates are insoluble in water.
 Source: chegg.com
Source: chegg.com
Amine can make hydrogen bonds with water. This is nitrobenzene, and it is a covalent compound which is a reason that it is soluble in benzene, alcohol etc but insoluble in water. The solubility rules tell you that all halides are soluble with the exception of those formed with siver, mercury, and lead ions.
 Source: bartleby.com
Source: bartleby.com
So here we�re taking a look at pb two plus and which of the following iron form compounds with pb two plus that will be generally soluble in water. Sodium chloride (nacl) table salt, or sodium chloride (nacl) , the most common ionic compound, is soluble in water (360 g/l). So according to rule six compound formed by s two minus and pb two plus will be insoluble.
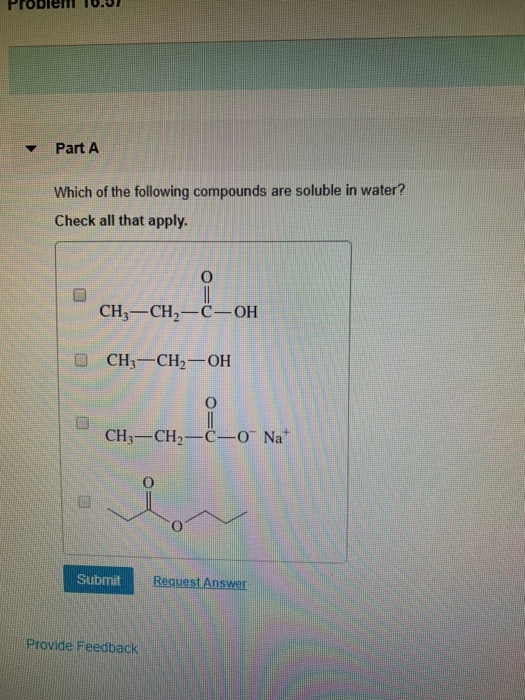 Source: chegg.com
Source: chegg.com
(iv) ethylene glycol is water soluble. Asked aug 7, 2021 in nursing by pockypokey. Amine which have less molecular mass are soluble in water.
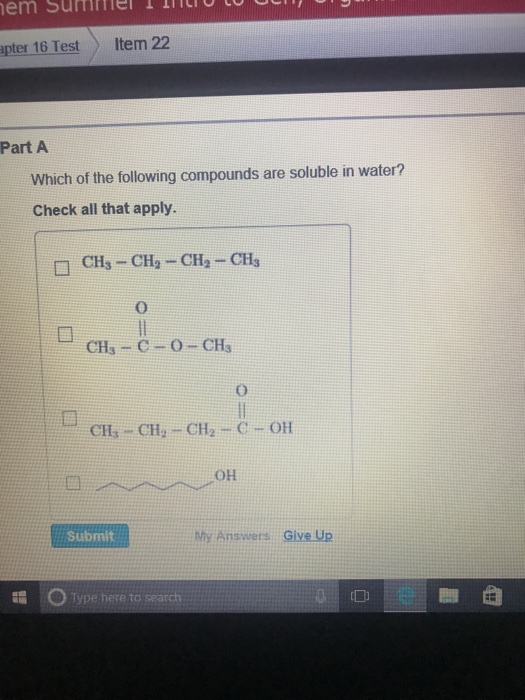
Dissociation of is as follows. It can react violently with iodine, hydrogen sulfide, or a mixture of barium oxide and air. There are several major types of compounds that are soluble in water.
 Source: lisbdnet.com
Source: lisbdnet.com
There are several major types of compounds that are soluble in water. Sodium carbonate () is soluble in water. Almost all ionic compounds are soluble in water but there are few exceptions.

The lattice enthalpy of agf is the lowest because of the least number of electon in its anion. Silver nitrate, or agn o3, is soluble because all nitrates are soluble, without exception. The lattric enthalpies of agbr and agl are even higher because of greater number of electrons in their anions.
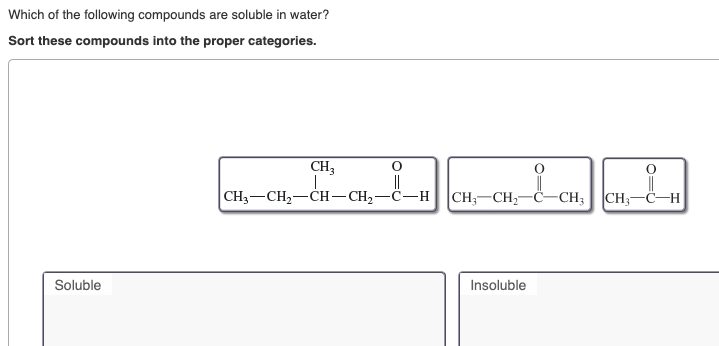
Amine can make hydrogen bonds with water. It can react violently with iodine, hydrogen sulfide, or a mixture of barium oxide and air. So, we can say c 6 h 5 n o 2 is soluble in benzene but almost insoluble in water.
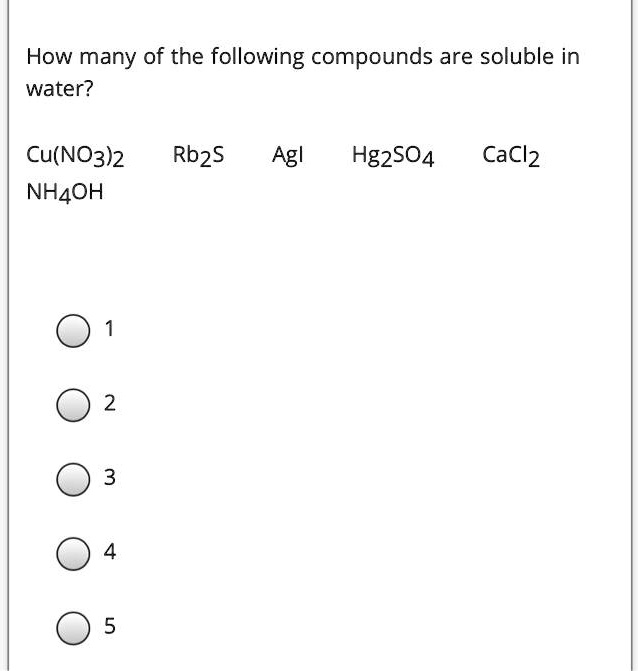 Source: numerade.com
Source: numerade.com
Ionic compounds are the compounds formed by transfer of electrons from one atom to another. So we need to reference our scalability rules. Option (b) is the correct answer.
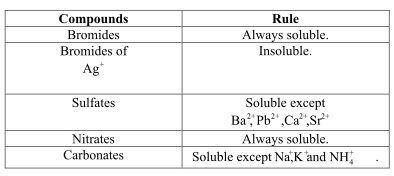 Source: ask.learncbse.in
Source: ask.learncbse.in
A) think about the following compounds: Tap card to see definition 👆. Lipid solubility which of the following compounds would you expect to be most soluble in water?
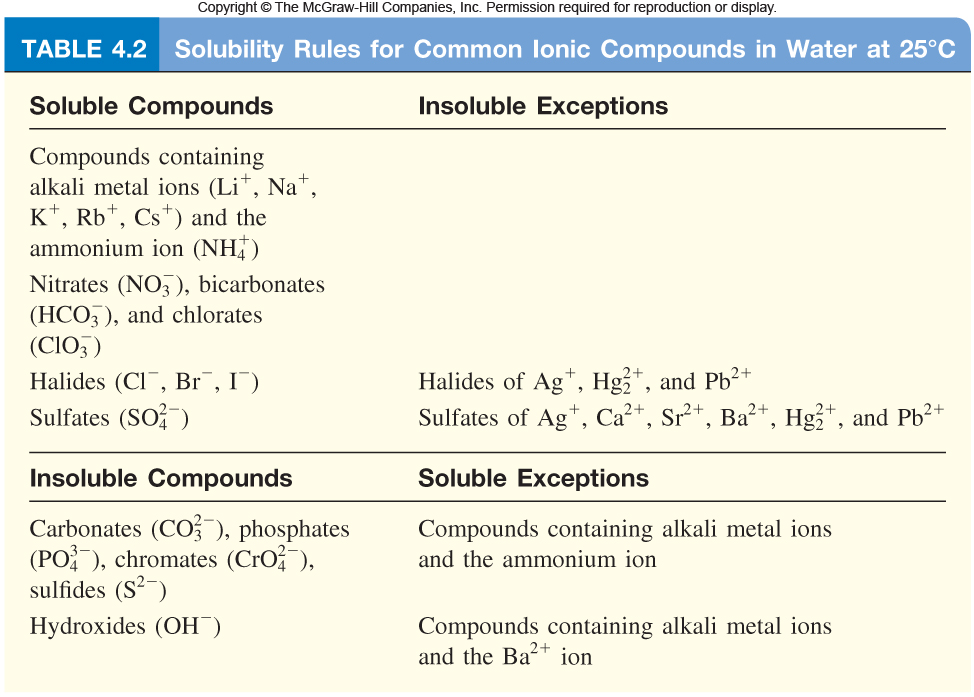 Source: socratic.org
Source: socratic.org
According to rule three, the compound formed by c l minus and pb. Lead chloride is also insoluble in cold water but is soluble in hot water. Iodine, cobalt (ii) chloride and sucrose (c12h22o11).
 Source: ask.learncbse.in
Source: ask.learncbse.in
Which of the following ionic compounds is most soluble in water? Lipid solubility which of the following compounds would you expect to be most soluble in water? * water is sometimes called the universal solvent. * water is good at dissolving ions and polar molecules, but poor at dissolving nonpolar molecules.
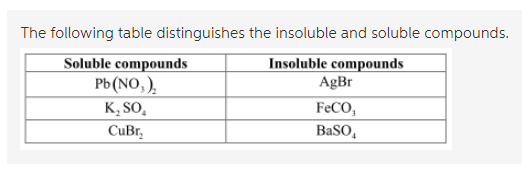 Source: ask.learncbse.in
Source: ask.learncbse.in
D) fecl3 eco, 7) if you had an aqueous mixture that contained as, k, and pb * cations, how many diff solids could precipitate if a chloride solution was added? (iv) ethylene glycol is water soluble. The lattice enthalpy of agf is the lowest because of the least number of electon in its anion.
Also Read :