Drag each item to the appropriate bin. Draw the lewis structure for the sulfite ion, so 3 2−.
Which Of The Following Are Valid Ionic Lewis Structures. The repulsion between these groups produce a linear shape for the molecule with bond angle of 180. Resonance hybrids contain delocalized electrons. In benzene, c 6 h 6, the six carbon atoms are arranged in a ring.two equivalent lewis structures can be drawn for benzene. In an ionic bond one atom looses all its outer electrons leaving behind a filled inner shell while another atom gains electrons to fill its valence shell.
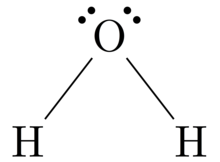 Lewis Structure - Wikipedia From en.wikipedia.org
Lewis Structure - Wikipedia From en.wikipedia.org
Related Post Lewis Structure - Wikipedia :
For the questions that follow, consider the best lewis structures of the following oxyanions: There are two lone pairs of electrons on the sulfur atom. Which of the following is a molecular. View available hint (e) reset help na ff] br ca br:]| |na:f:
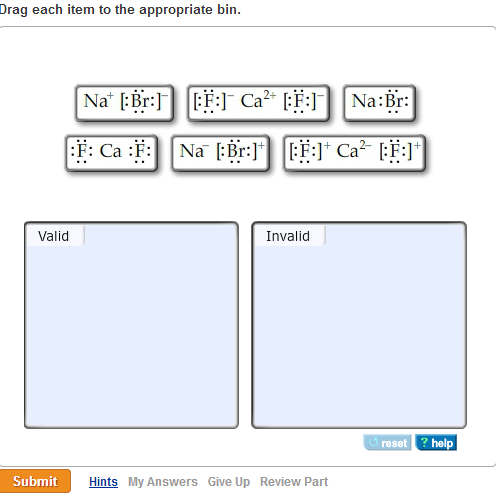
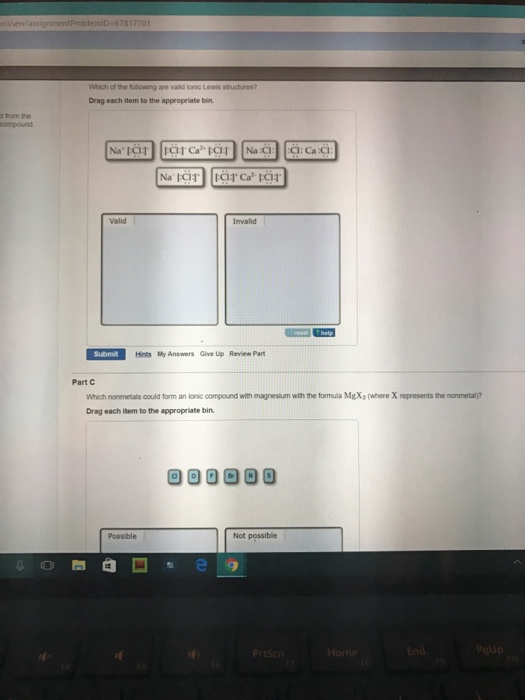
M which of the following are valid ionic lewis structures?
M which of the following are valid ionic lewis structures? There are two lone pairs of electrons on the sulfur atom. Which is the net ionic equation for the reaction? Which of the following is a valid c++ array definition; Drag each item to the appropriate bin. We will use this later in the class to make very reactive reagents.
 Source: clutchprep.com
Source: clutchprep.com
Which of the following are valid ionic lewis structures? Drag each item to the appropriate bins. For the questions that follow, consider the best lewis structures of the following oxyanions:
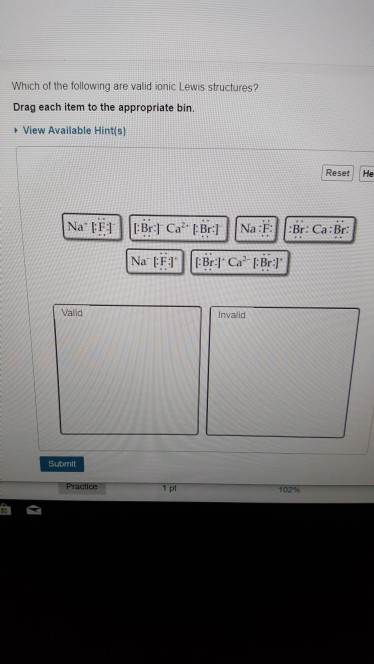
Which of the following are valid ionic lewis structures? Which of the statements below is true for the lewis structure of the sulfite ion?a) there must be a double bond between the sulfur atom and one of the oxygen atoms to ensure that all atoms have an octet (eight electrons).b) there are no lone pairs of electrons on the sulfur atom.c) there is one lone pair of electrons on the sulfur. In lewis structures, the goal is to make almost all atoms have this structure.
 Source: oneclass.com
Source: oneclass.com
Which of the following statements is true? Represent the following ionic compounds by lewis structures: Which of the following is.
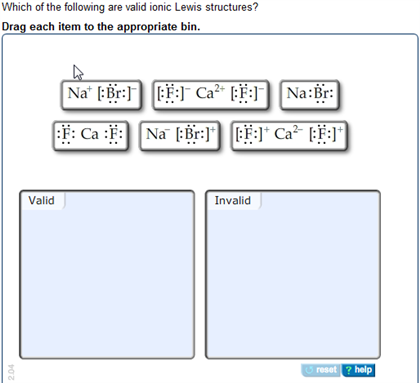 Source: chegg.com
Source: chegg.com
For example, co2, with the lewis structure shown below, has two electron groups (two double bonds) around the central atom. S, electrons are transferred esulting in the formation of which of the following are valid ionic lewis structures? In an ionic bond one atom looses all its outer electrons leaving behind a filled inner shell while another atom gains electrons to fill its valence shell.
![Solved] Which Of The Following Are Valid Ionic Lewis Structures? | Course Hero](https://www.coursehero.com/qa/attachment/22899122/ “Solved] Which Of The Following Are Valid Ionic Lewis Structures? | Course Hero”) Source: coursehero.com
Which of the following is a valid selector for a class named menu? Which of the following are valid ionic lewis structures? In an ionic bond one atom looses all its outer electrons leaving behind a filled inner shell while another atom gains electrons to fill its valence shell.
![Solved:which Of The Following Are Valid Ionic Lewis Structures Drag Each Item To The Appropriate Bin: View Avallable Hlnt(S) Reset (Help Na" [Ci;] Fci:] Ca? [Ci:] Na :Ci: Ci: Ca:ci: Na- [Ci] Source: numerade.com
Which of the following are valid ionic lewis structures? Which of the following are valid ionic lewis structures? A valid lewis structure of _____ cannot be drawn without violating the octet rule.
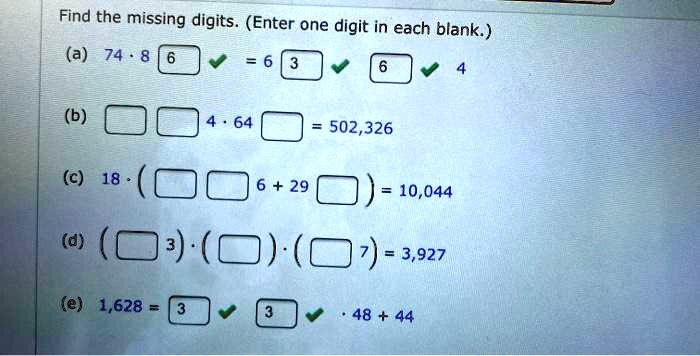 Source: itprospt.com
Source: itprospt.com
Resonance structures differ in the arrangement of electrons but not in the arrangement of atoms. There are single bonds between the sulfur atom and each of the oxygen atoms b. Which of the following are valid ionic lewis structures?
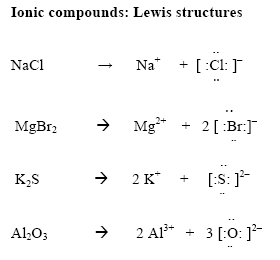 Source: kentchemistry.com
Source: kentchemistry.com
For the questions that follow, consider the best lewis structures of the following oxyanions: Resonance structures differ in the arrangement of electrons but not in the arrangement of atoms. Which of the following are valid ionic lewis structures?
 Source: oneclass.com
Source: oneclass.com
Drag each item to the appropriate bins. Which of the following are valid ionic lewis structures?. In both structures, the carbon atoms have a trigonal planar geometry.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
Which of the following are valid ionic lewis structures? Valid invalid submit macbook air. Which of the following are valid ionic lewis structures?
 Source: youtube.com
Source: youtube.com
The repulsion between these groups produce a linear shape for the molecule with bond angle of 180. Which of the following are valid ionic lewis structures? Write a plausible lewis structure for crotonaldehyde, ch3chchcho, a substance used in tear gas and insecticides.
 Source: chegg.com
Source: chegg.com
In benzene, c 6 h 6, the six carbon atoms are arranged in a ring.two equivalent lewis structures can be drawn for benzene. Represent the following ionic compounds by lewis structures: Resonance hybrids contain delocalized electrons.

Draw the lewis structure for the sulfite ion, so 3 2−. Which of the following are valid ionic lewis structures? Ca:br na ef br* ca2 :br:
 Source: youtube.com
Source: youtube.com
There are single bonds between the sulfur atom and each of the oxygen atoms b. There are single bonds between the sulfur atom and each of the oxygen atoms b. In benzene, c 6 h 6, the six carbon atoms are arranged in a ring.two equivalent lewis structures can be drawn for benzene.
 Source: clutchprep.com
Source: clutchprep.com
Which of the following are valid ionic lewis structures?. Drag each item to the appropriate bin. Draw the lewis structure for the sulfite ion, so 3 2−.
 Source: clutchprep.com
Source: clutchprep.com
Which nonmetals could form an ionic compound with magnesium with the formula mgx2 (where x represents the nonmetal)? Drawing lewis structures where one or more atoms does not have a full valence shell. Drag each item to the appropriate bin.
 Source: en.wikipedia.org
Source: en.wikipedia.org
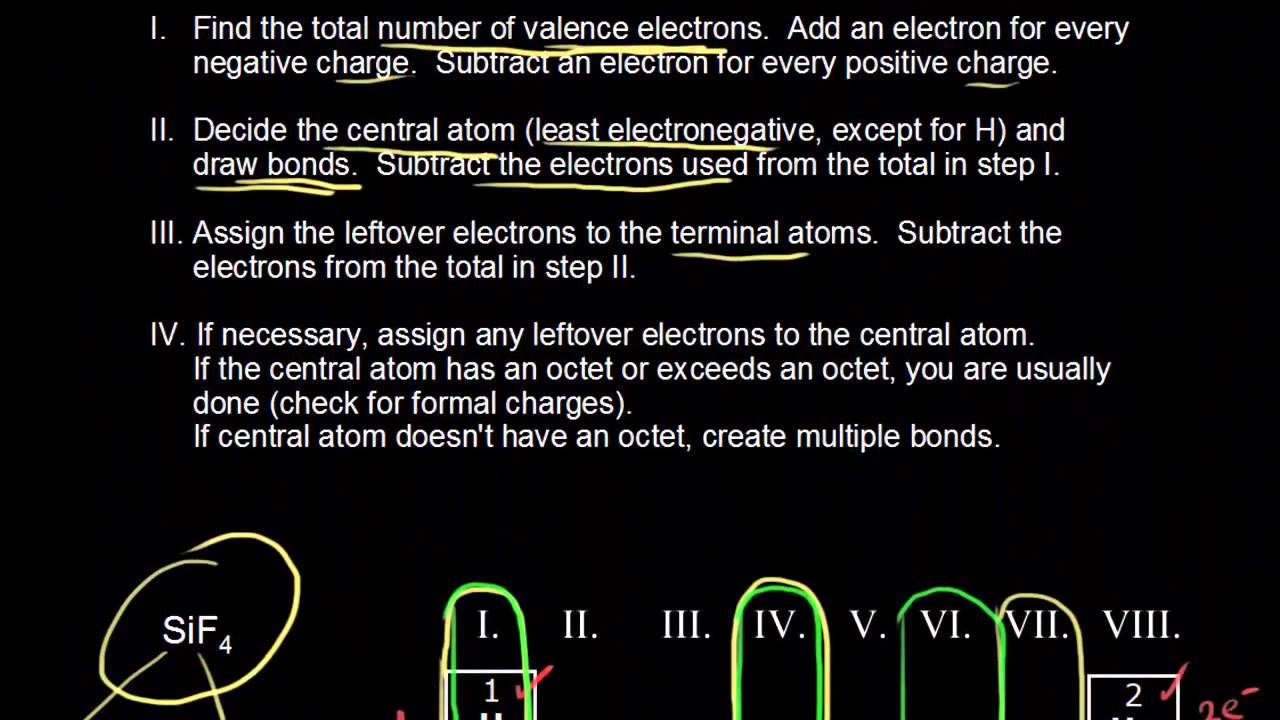
Which of the statements below is true for the lewis structure of the sulfite ion?a) there must be a double bond between the sulfur atom and one of the oxygen atoms to ensure that all atoms have an octet (eight electrons).b) there are no lone pairs of electrons on the sulfur atom.c) there is one lone pair of electrons on the sulfur. Which of the following is. In the lewis structures listed here m and x represent various elements in the third period of the periodic table.
In an ionic bond one atom looses all its outer electrons leaving behind a filled inner shell while another atom gains electrons to fill its valence shell. In lewis structures, the goal is to make almost all atoms have this structure. Which of the following are valid ionic lewis structures?
 Source: khanacademy.org
Source: khanacademy.org
Drag each item to the appropriate bin. In an ionic bond one atom looses all its outer electrons leaving behind a filled inner shell while another atom gains electrons to fill its valence shell. Which of the following is not a valid c++ identifier?
 Source: shimizu-uofsc.net
Source: shimizu-uofsc.net
Drag each item to the appropriate bins. Resonance structures for a given compound always contribute equally to the resonance hybrid. Which is the net ionic equation for the reaction?
Also Read :





