We all know that as the chain length of an alkane increases the boiling point of the alkanes also increases due to higher molecular weight. As explained, since there is a bigger volume to an alkane than its corresponding alkyne (i.e.
Which Of The Following Alkanes Has The Highest Boiling Point. As the number of carbon atom increases, the boiling points of alkane increases because van der waal�s forces increases. B) alkanes can form chains or cyclic structures. (2) ethylene glycol contains two carbon atoms and two hydroxyl. But, the forces in hexane will be stronger than those in butane because hexane molecules are larger and therefore capable of more extensive dispersion forces between each other than the molecules of butane.
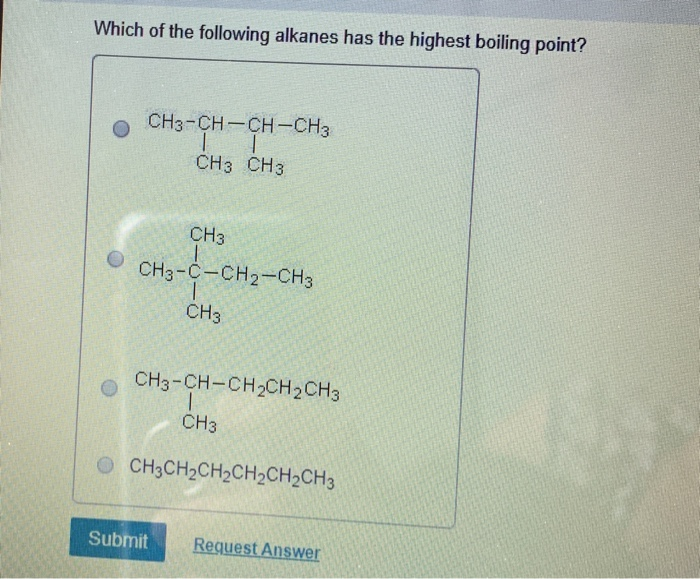 Solved Which Of The Following Alkanes Has The Highest | Chegg.com From chegg.com
Solved Which Of The Following Alkanes Has The Highest | Chegg.com From chegg.com
Related Post Solved Which Of The Following Alkanes Has The Highest | Chegg.com :
D) alkanes are more dense than water. (2) ethylene glycol contains two carbon atoms and two hydroxyl. B) alkanes can form chains or cyclic structures. However, there�s something else in play here:
Ch4 = nonpolar molecule = london forces = lowest boiling point.
Which of the following alkanes has the highest boiling point? It is obvious that base on the data above, the alkene that has the highest boiling point is hexane which boils at 68 degrees celcius. So, 1−chloro−pentane has the highest boiling point. Maximum boiling azeotropes are those which have the boiling point higher than any of its constituents. C4h10 d.c6h14 e.c8h18 indicate which of the following molecules exhibits the greatest dispersion forces о а.ch4 ob.ch3ch2ch2ch3 с. C5h12 o b.c7h16 o c.
 Source: toppr.com
Source: toppr.com
Howerver, branching result in decrease in boiling point due to decreased surface area which results in weaker van der waal�s forces. Why does hexane have a high boiling point? Nonane will have a higher boiling point than octane, because it has a longer carbon chain than octane.
 Source: toppr.com
Source: toppr.com
Which of the following has maximum boiling point? In the case of melting point, alkanes containing even no. Nonane will have a higher boiling point than octane, because it has a longer carbon chain than octane.
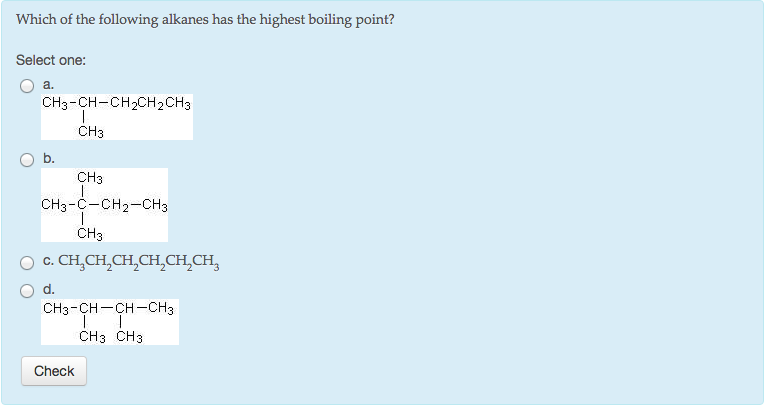 Source: chegg.com
Source: chegg.com
So, 1−chloro−pentane has the highest boiling point. Which of the following compounds has the highest boiling point ?class:11subject: C4h10 d.c6h14 e.c8h18 indicate which of the following molecules exhibits the greatest dispersion forces о а.ch4 ob.ch3ch2ch2ch3 с.

(1) alcohols have higher boiling points than alkanes of similar molecular mass because of hydrogen bonding. However, there�s something else in play here: (2) ethylene glycol contains two carbon atoms and two hydroxyl.
 Source: askiitians.com
Source: askiitians.com
In the case of melting point, alkanes containing even no. C) alkanes that contain only carbon and hydrogen atoms have low water solubility. Ch4 = nonpolar molecule = london forces = lowest boiling point.
 Source: chegg.com
Source: chegg.com
Subsequently, one may also ask, do alkanes have higher boiling points? Boiling point of alkane increases with molar mass. Ethanol contains oxygen and hydrogen can form hydrogen bonds which require more energy to be broken than the van der waals forces in ethanethiol.
 Source: formsbirds.com
Source: formsbirds.com
Nonane will have a higher boiling point than octane, because it has a longer carbon chain than octane. Nonane will have a higher boiling point than octane, because it has a longer carbon chain than octane. Nonane will have a higher boiling point than octane, because it has a longer carbon chain than octane.
 Source: youtube.com
Source: youtube.com
In the case of melting point, alkanes containing even no. Which of the following alkane has the lowest boiling point and highest melting point? D) alkanes are more dense than water.

So, 1−chloro−pentane has the highest boiling point. What is maximum boiling azeotrope? C4h10 d.c6h14 e.c8h18 indicate which of the following molecules exhibits the greatest dispersion forces о а.ch4 ob.ch3ch2ch2ch3 с.
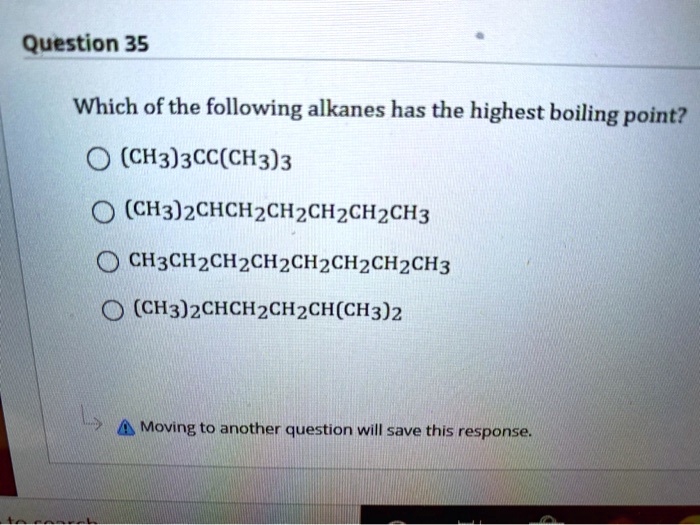 Source: numerade.com
Source: numerade.com
What is maximum boiling azeotrope? Again, the boiling points of the branched chain alkanes are less than the straight chain isomers. In the case of melting point, alkanes containing even no.
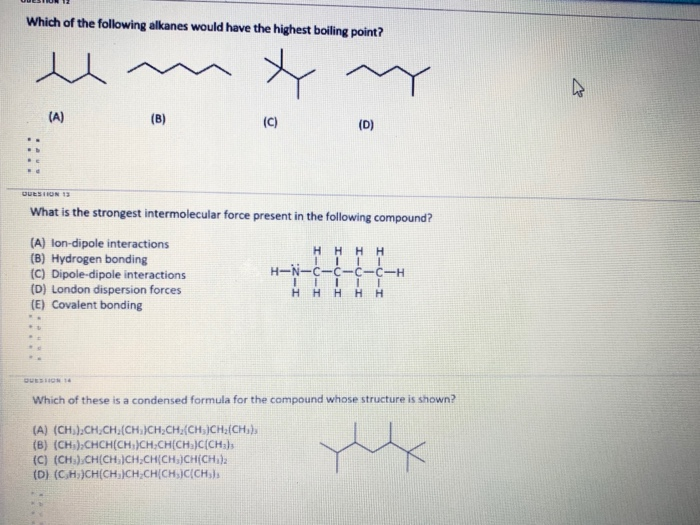 Source: chegg.com
Source: chegg.com
With the same number of carbons) the alkane should have a higher boiling point. Subsequently, one may also ask, do alkanes have higher boiling points? Which of the following alkanes has the highest boiling point?
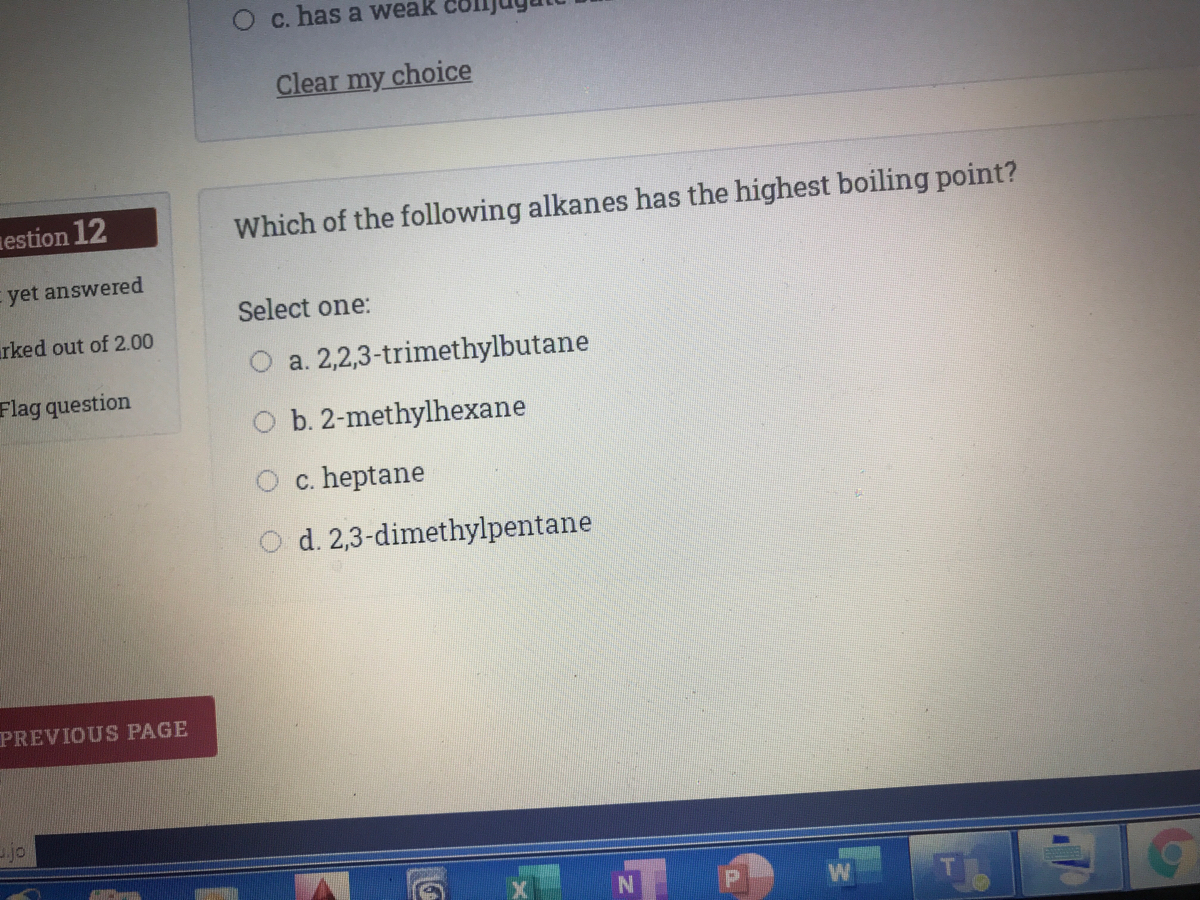 Source: bartleby.com
Source: bartleby.com
(a) what effect the branching of an alkane has on its melting point? (2) ethylene glycol contains two carbon atoms and two hydroxyl. C5h12 o b.c7h16 o c.
 Source: numerade.com
Source: numerade.com
However, there�s something else in play here: Ch4 = nonpolar molecule = london forces = lowest boiling point. As explained, since there is a bigger volume to an alkane than its corresponding alkyne (i.e.
 Source: brainly.in
Source: brainly.in
Hence the boiling point is low. (1) alcohols have higher boiling points than alkanes of similar molecular mass because of hydrogen bonding. C 2 h 6 c 3 h 8 c 4 h 10.
 Source: youtube.com
Source: youtube.com
Why does hexane have a high boiling point? Which butane has the minimum boiling point? As explained, since there is a bigger volume to an alkane than its corresponding alkyne (i.e.
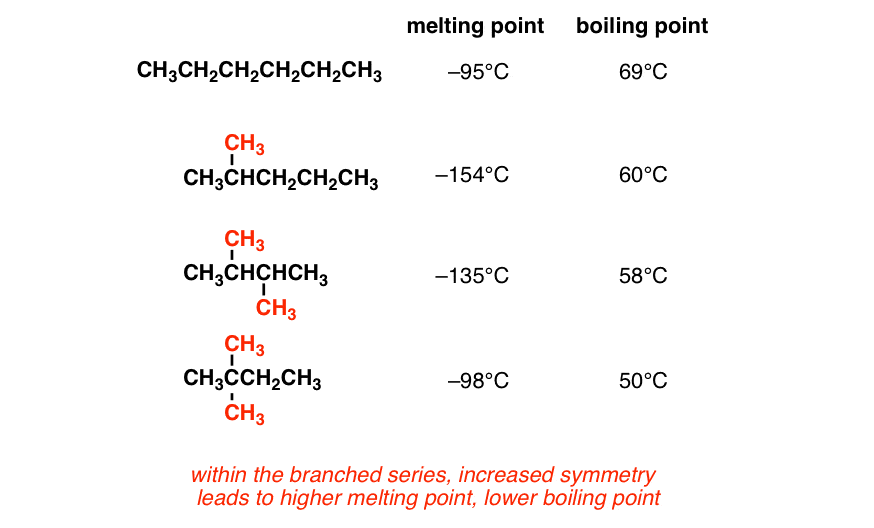 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
A) alkanes are hydrocarbons that contain only single bonds. Maximum boiling azeotropes are those which have the boiling point higher than any of its constituents. C 2 h 6 c 3 h 8 c 4 h 10.
 Source: study.com
Source: study.com
Boiling point of alkane increases with molar mass. In the case of melting point, alkanes containing even no. As the number of carbon atom increases, the boiling points of alkane increases because van der waal�s forces increases.
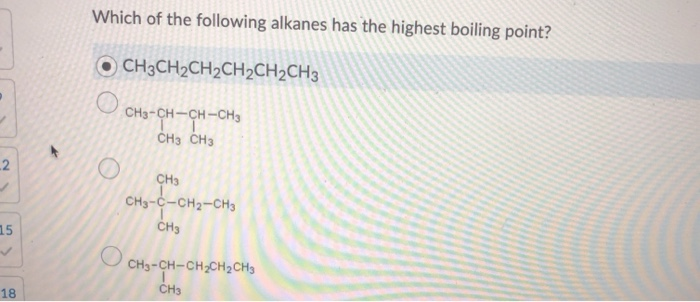 Source: chegg.com
Source: chegg.com
Octane will have a higher boiling point than 2,2,3,3‑tetramethylbutane, because it branches less than 2,2,3,3‑tetramethylbutane, and therefore has a larger “surface area” and more van der waals forces. What is maximum boiling azeotrope? Ans.(a) in general conception, as the branching increases packing of the molecules in the crystals becomes less close.
 Source: chegg.com
Source: chegg.com
What is maximum boiling azeotrope? Closed sep 29 by urmillasahu. Octane will have a higher boiling point than 2,2,3,3‑tetramethylbutane, because it branches less than 2,2,3,3‑tetramethylbutane, and therefore has a larger “surface area” and more van der waals forces.

Hence the boiling point is low. Which of the following has maximum boiling point? Among isomeric alkanes, branching decreases boiling point.
Also Read :





