By signing up, you'll get. This image suggests a bond angle of 106.7∘, which apparently is the smallest here.
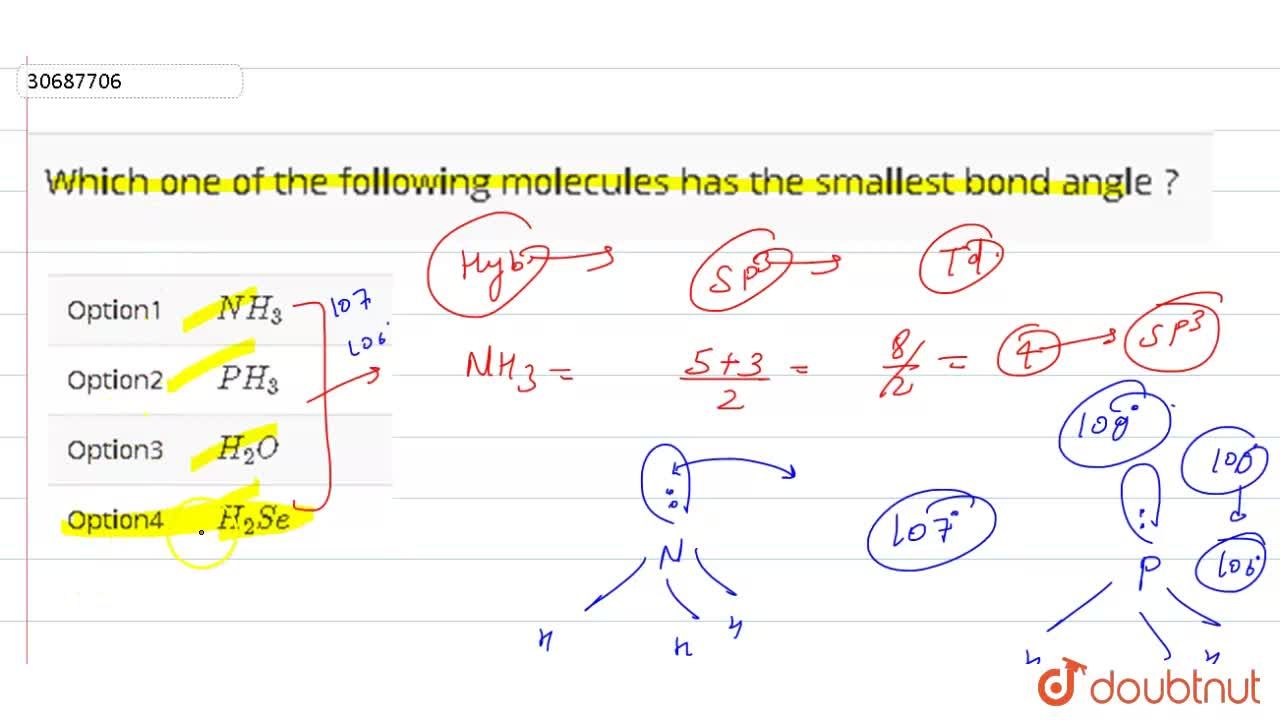
Which Molecule Has The Smallest Bond Angle. B) n h3 is sp3 hybridized and it has pyrimidal shape. Which of the following molecules has smallest bond angle: Bond angle is the angle formed by two connected atoms and the central atom. Hence, the correct option is a.

Related Post Which One Of The Following Compounds Has The Smallest Bond Angle? (A) So2 (B) Oh2 (C) Sh2 (D) Nh3 - Sarthaks Econnect | Largest Online Education Community :
A) ch4 is sp3 hybridized and has a regular tetrahedral structure with a bond angle of 109∘. As the size of central atom increases lone pair bond pair repulsion increases so, bond angle decreases. Hybridisation involved in n h 3 is s p 3 with one lone pair, in b e f 2 is s p 2 with no lone pair, in h 2 o is s p 3 with two lone pairs, in c h 4 is s p 3 with no lone pair, in s o 2 is s p 1 with one lone pair. June 28, 2021 by admin.
So this bond angle in h2 is equal to 104.5° in cell phone.
(a) nh3 (b) so2 (c) h2o (d) h2s $\ce{h2o}$ and $\ce{nh3}$ are hydrides of the same period so we can use the first rule to determine that $\ce{h2o}$ has a smaller bond angle. Which of the following molecules are polar: The possibility to make a mistake is that you may choose option d. The bond angles in trigonal planar molecules are larger than those in tetrahedral molecules. Hence, the correct option is a.
 Source: doubtnut.com
Source: doubtnut.com
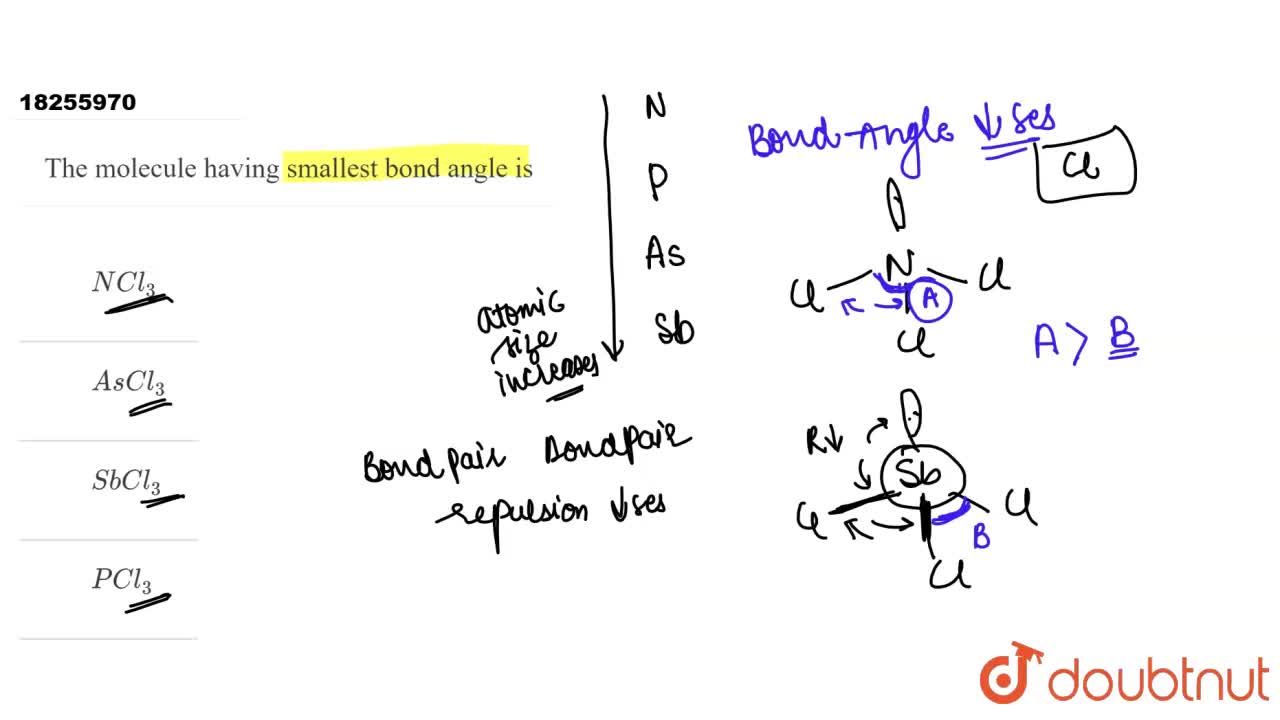
Which of the following molecules has the smallest bond angle between any two hydrogen atoms? The molecule having smallest bond angle is. Hybridisation involved in n h 3 is s p 3 with one lone pair, in b e f 2 is s p 2 with no lone pair, in h 2 o is s p 3 with two lone pairs, in c h 4 is s p 3 with no lone pair, in s o 2 is s p 1 with one lone pair.

Where is the central atom located in a trigonal bipyramidal molecule? S o 2 , s o 3 , c o 2 , n o 3 , or h 2 o ? Which one of the following molecules has.
So it has smallest bond adam. Learn this topic by watching bond angles concept videos. But down the group as.
(a) nh3 (b) so2 (c) h2o (d) h2s In the series of given molecules of ascl3, sbcl3, pcl3 and ncl3 the lowest bond angle is observed in sbcl3. E x e r c i s e 3.
 Source: clutchprep.com
Source: clutchprep.com
Ccl4, cl2, hcl, and kcl? Which of the following molecule. Which one of the following compounds has the smallest bond angle in its molecule ?
 Source: toppr.com
Source: toppr.com
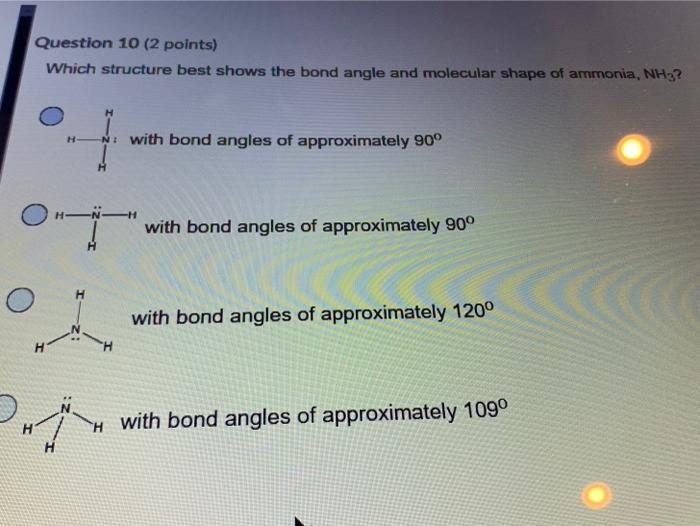
Assume that you must determine the bond angles in bf_3. By signing up, you�ll get. On the other hand, nh3 has a lone pair of electrons, which compresses the three n−h bonds down due to charge repulsions between the lone pair and the bonding pairs:
 Source: toppr.com
Source: toppr.com
Which of the following molecules has the lowest bond angle? The possibility to make a mistake is that you may choose option d. In these situations the molecule with the most electronegative central atom has the largest bond angles.
 Source: toppr.com
Source: toppr.com
6 which of the following molecules has the smallest bond angle? $\ce{h2o}$ and $\ce{nh3}$ are hydrides of the same period so we can use the first rule to determine that $\ce{h2o}$ has a smaller bond angle. This is because as we move down the group the size of the central atom increases and the force of attractions between the atoms increases hence.

Based on electron geometries, which of the following molecules has the smallest bond angle between any two adjacent hydrogen atoms? Which of the following molecules has smallest bond angle: There are three basic steps to determining the bond angles in a molecule:
 Source: youtube.com
Source: youtube.com
It may also be defined as the angle between two side atoms and the central atom. The bond angles formed are close to 90°. So this bond angle in h2 is equal to 104.5° in cell phone.
 Source: doubtnut.com
Source: doubtnut.com
It may also be defined as the angle between two side atoms and the central atom. (a) nh3 (b) so2 (c) h2o (d) h2s On the other hand, nh3 has a lone pair of electrons, which compresses the three n−h bonds down due to charge repulsions between the lone pair and the bonding pairs:
 Source: clutchprep.com
Source: clutchprep.com
Ccl4, cl2, hcl, and kcl? Which of the following molecules are polar: Hence, the correct option is a.

Write the lewis dot structure for the molecule. Which one of the following compounds has the smallest bond angle in its molecule ? What is the bond angle for if5?, the molecular geometry of if5 is square pyramidal.
 Source: doubtnut.com
Source: doubtnut.com
A h2o b nh3 c ch4 d sf6 Bond angle is the angle formed by two connected atoms and the central atom. It may also be defined as the angle between two side atoms and the central atom.
 Source: youtube.com
Source: youtube.com
Which of the following compounds has polar covalent bonds: What is the molecular geometry of brf5?, the molecular geometry of brf 5 is square pyramidal with asymmetric charge distribution on the central atom. Assume that you must determine the bond angles in bf_3.
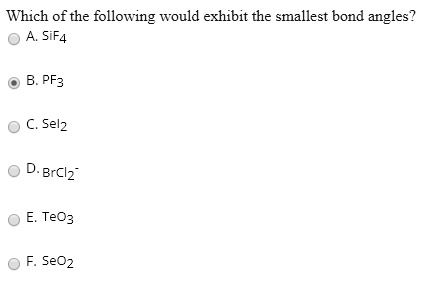 Source: numerade.com
Source: numerade.com
And we know that as the size of the atom increases, bond length increases and bond angle decreases. In the series of given molecules of ascl3, sbcl3, pcl3 and ncl3 the lowest bond angle is observed in sbcl3. This hydrogen cell for hydrogen bond angles 92.5° and this bond angle between hydrogen selenium and hydrogen is 91 daily.
 Source: targetbatch.com
Source: targetbatch.com
As the size of central atom increases lone pair bond pair repulsion increases so, bond angle decreases. Which of the following compounds has polar covalent bonds: Asked jun 29, 2017 in chemistry by majormask.
 Source: slideplayer.com
Source: slideplayer.com
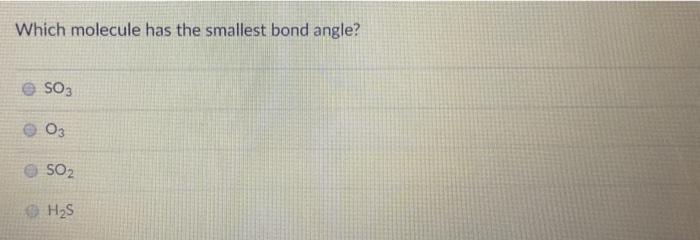
The bond angle in cl20 is expected to be approximately. Hence, the correct option is a. Which molecule has the smallest bond angles?
 Source: slideplayer.com
Source: slideplayer.com
This is because as we move down the group the size of the central atom increases and the force of attractions between the atoms increases hence. The bond angles in trigonal planar molecules are larger than those in tetrahedral molecules. Assume that you must determine the bond angles in bf_3.
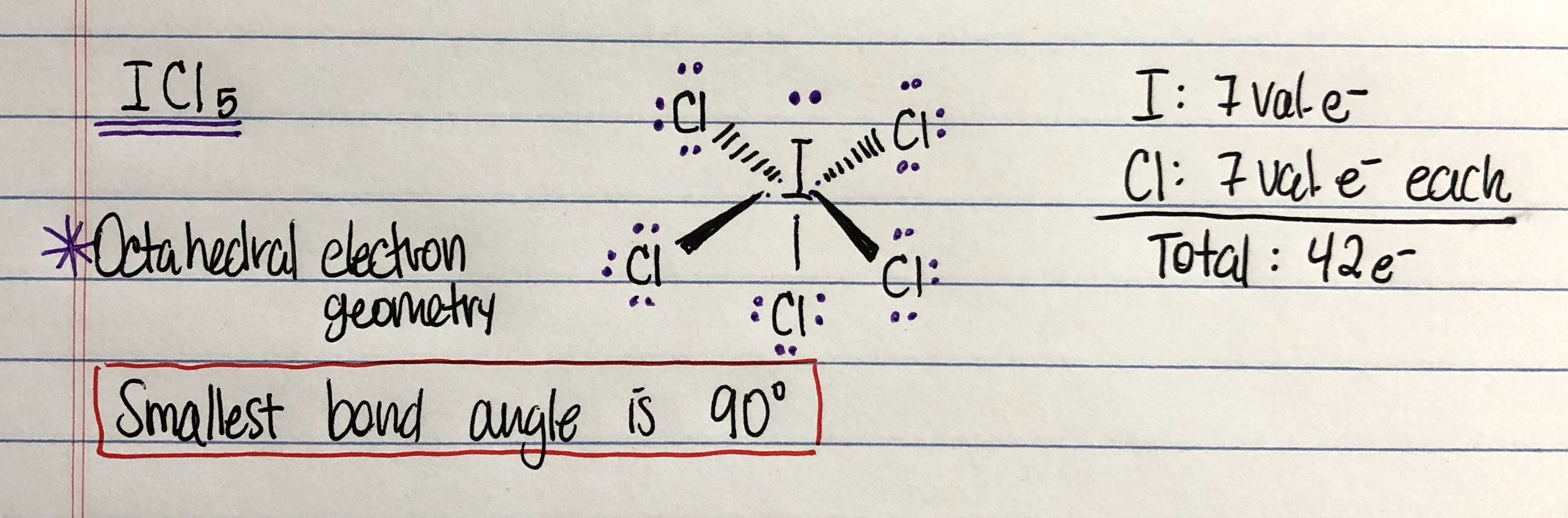 Source: clutchprep.com
Source: clutchprep.com
So it has smallest bond adam. It may also be defined as the angle between two side atoms and the central atom. In the series of given molecules of ascl3, sbcl3, pcl3 and ncl3 the lowest bond angle is observed in sbcl3.
Also Read :