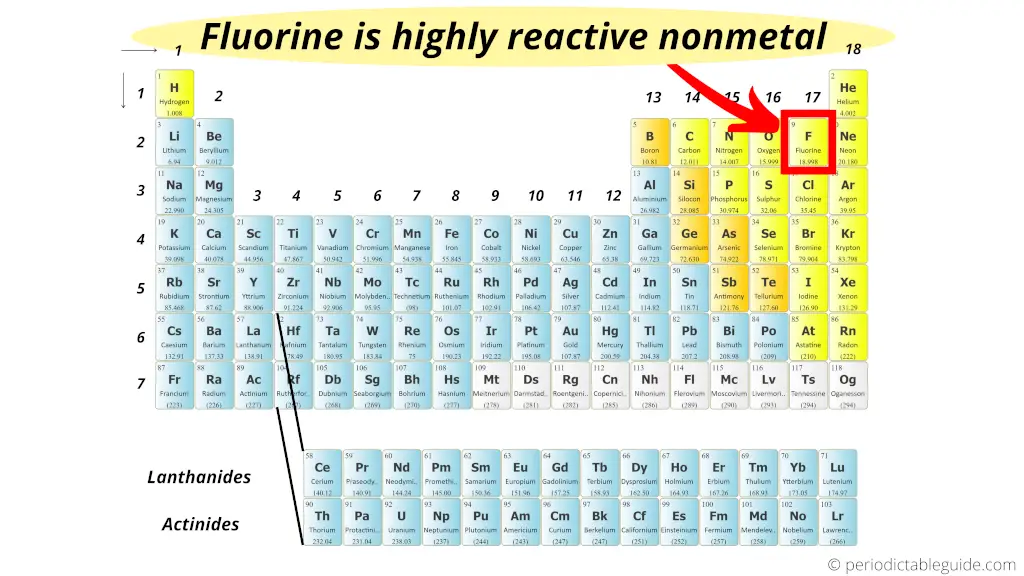
Fluorine is a halogen, which is group 17 on the periodic table, and the halogens are the most reactive nonmetals. Nov 172:54 pm nonmetal reactivity fluorine has the highest electronegativity and the smallest atomic radius therefore it gains valence electrons the most easily.
Which Is The Most Reactive Nonmetal. Due to its strong electro negativity & small size, fluorine has a strong tendency to accept electrons from other atoms or ions. So cesium is the most reactive element in group 1 and whole the perodic table. Fluorine has the highest electronegativity and the smallest atomic radius therefore it gains valence electrons the most easily. The most reactive nonmetal is fluorine.
 What Is The Most Reactive Nonmetal And Why? Faqs On Non-Metals From unlimitededu.net
What Is The Most Reactive Nonmetal And Why? Faqs On Non-Metals From unlimitededu.net
Related Post What Is The Most Reactive Nonmetal And Why? Faqs On Non-Metals :
Which element in period 2 of the periodic table is the most reactive nonmetal? Due to its strong electro negativity & small size, fluorine has a strong tendency to accept electrons from other atoms or ions. Which element is the most reactive nonmetal. Fluorine is the only element that reacts with xenon of viii a group, due to its high electronegativity value.
It is not found in nature as a free element.
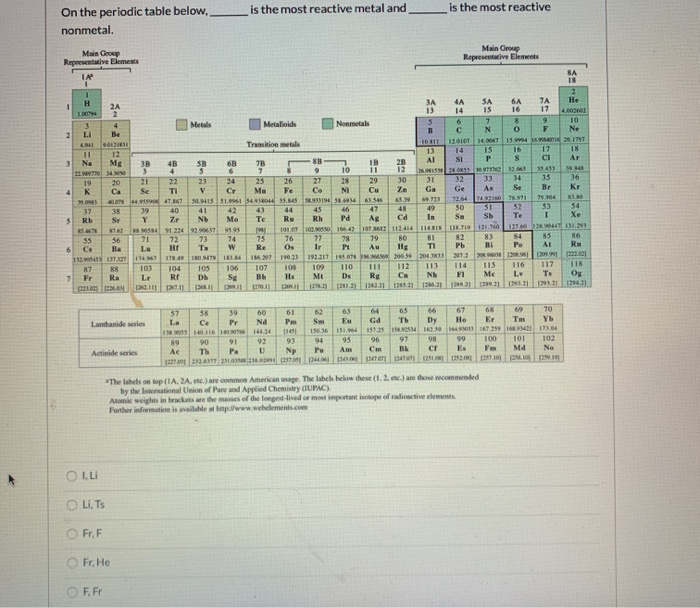
The most reactive nonmetal is fluorine, f. Fluorine is the most reactive nonmetal because it is the most electronegative nonmetal in the periodic table. Fluorine is the most reactive nonmetal. > chlorine is the most sensitive metal of the halogen family, for example chlorine, bromine, iodine and fluorine. In the metals, reactivity increases down a group and to the right. The most reactive nonmetal is:

Reactivity of metals is based on processes such as the formation of halide compounds with halogens and how easily they displace hydrogen from dilute acids. Since the noble gases are a special group because of their lack of reactivity, the element fluorine is the most reactive nonmetal. [139] in periodic table terms, the counterparts of the highly nonmetallic halogens, in group 17 are the highly reactive metals, such as sodium and potassium, in.
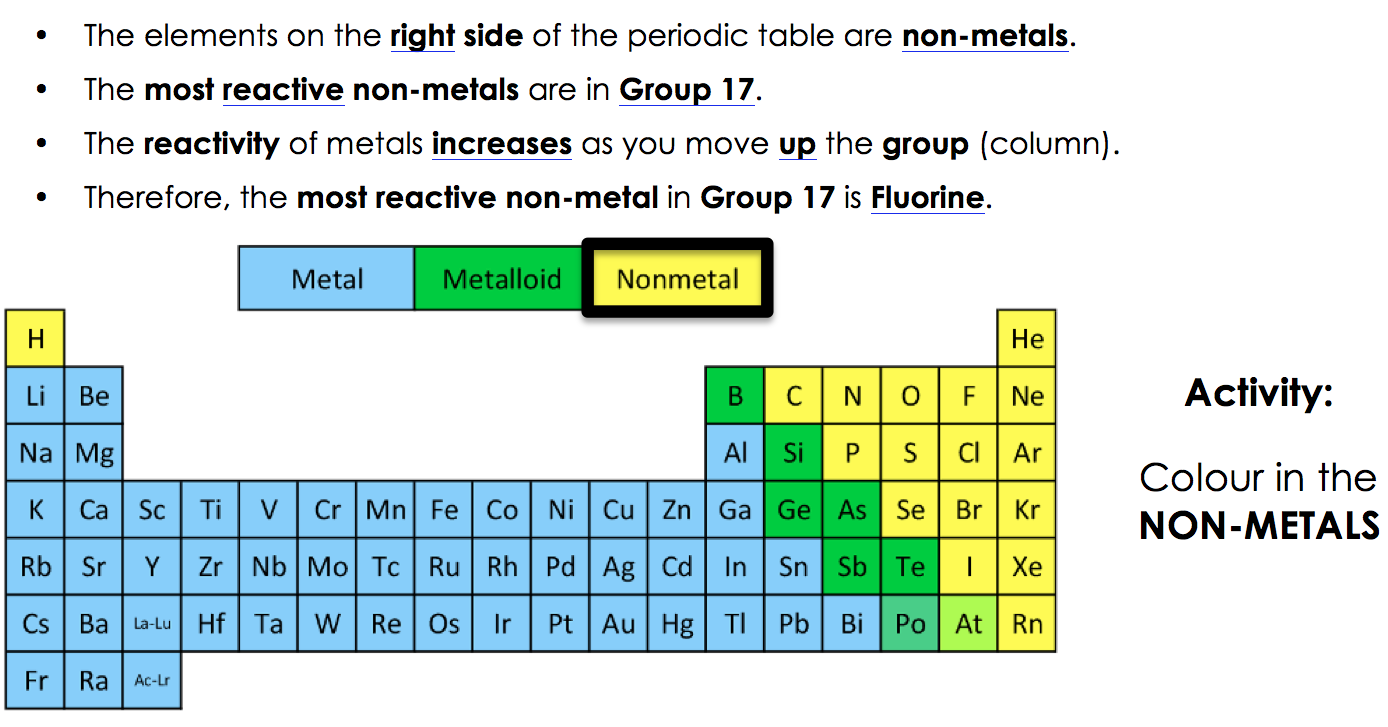 Source: willowwoodlessons.weebly.com
Source: willowwoodlessons.weebly.com
What are the 2 most reactive nonmetals? This is because they all have one empty space in their valence electron shells. Krypton is a halogen, so it should be kept secret because it is so reactive and toxic.
 Source: slideplayer.com
Source: slideplayer.com
Which non metal is highly reactive towards air? What are the 2 most reactive nonmetals? The most reactive nonmetal is fluorine.
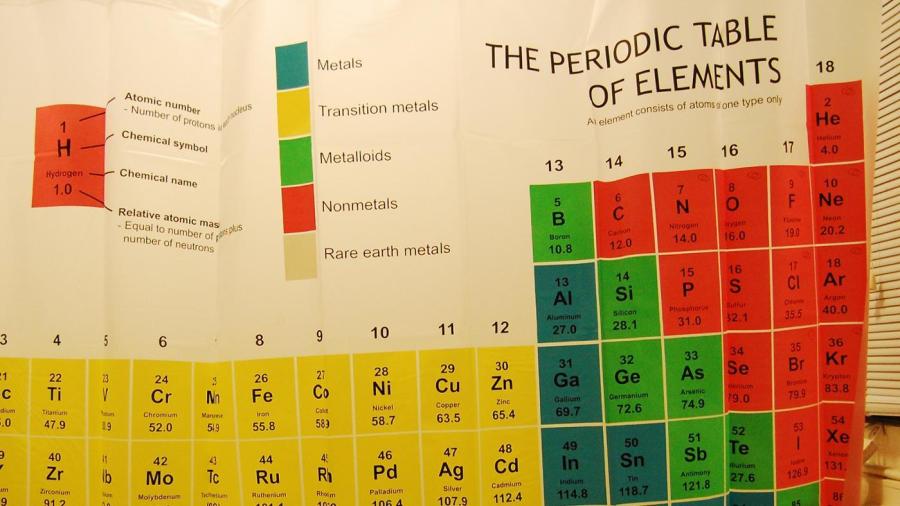 Source: reference.com
Source: reference.com
The reactivity of the alkali metals increases down thegroup. The group 1 elements in the periodic table are known as the alkali metals. Potassium is in the most reactive group of elements, the alkali metals, but it’s not the most reactive metal within the group.
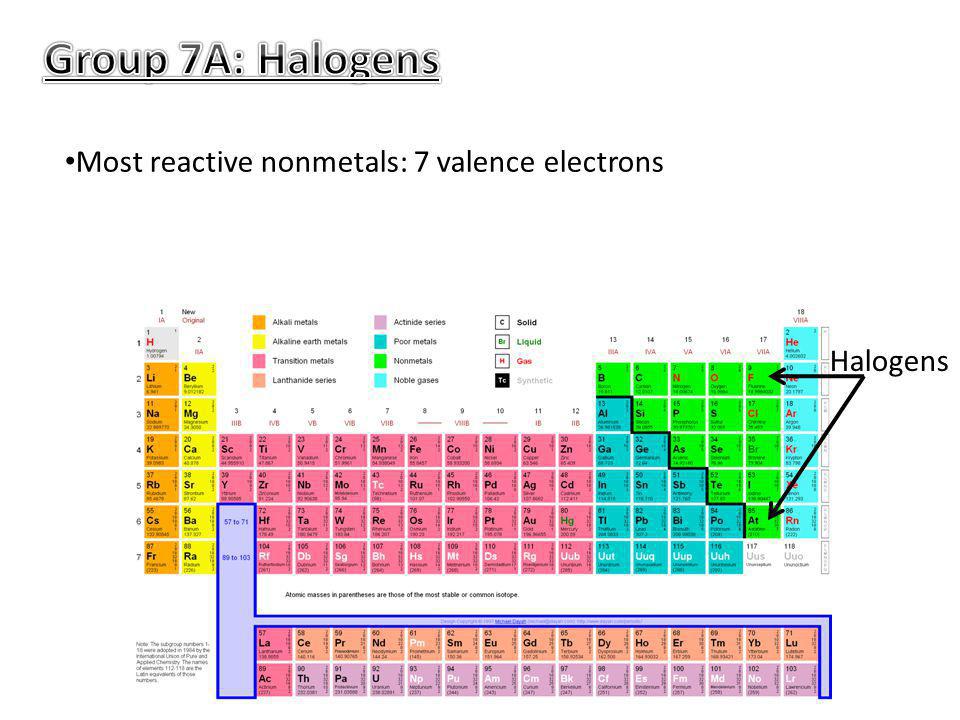 Source: slideplayer.com
Source: slideplayer.com
Then, which is more reactive metal or nonmetal? Which non metal is highly reactive towards air? This is because they all have one empty space in their valence electron shells.
 Source: brainly.com
Source: brainly.com
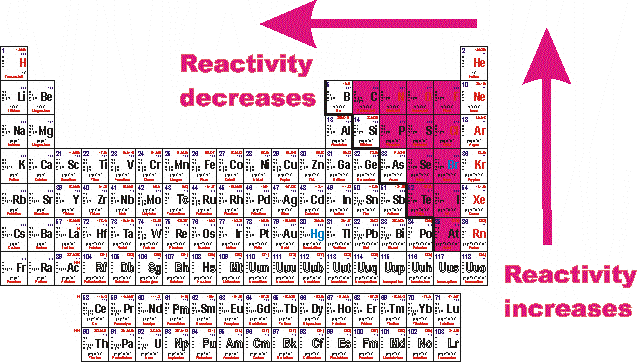
The trend in reactivity in the nonmetals is the opposite of the trend in the metals. Fluorine has the highest electronegativity and the smallest atomic radius therefore it gains valence electrons the most easily. The reactive and strongly electronegative nature of the nonmetal halogens represents the epitome of nonmetallic character.
 Source: unlimitededu.net
Source: unlimitededu.net
What are the most reactive metal and nonmetal? Hereof, what is the most reactive nonmetal and why? Since the noble gases are a special group because of their lack of reactivity, the element fluorine is the most reactive nonmetal.

Fluorine is the most reactive element in this group. This means it pulls you electrons towards it stronger than any other element. It is not found in nature as a free element.
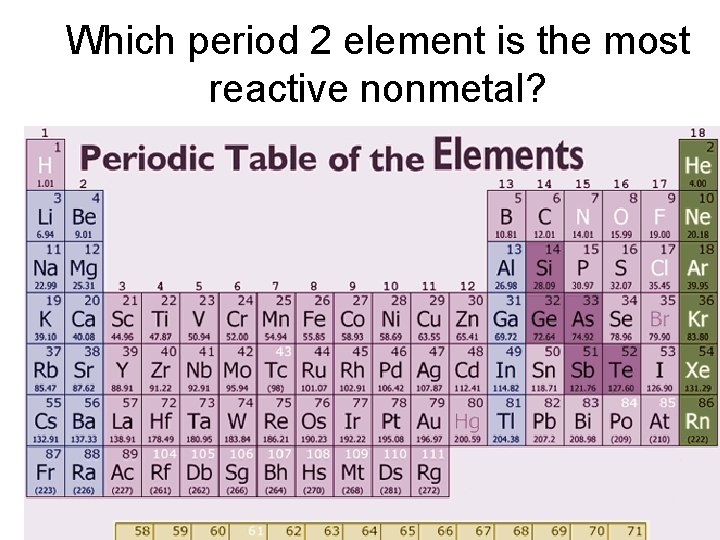 Source: slidetodoc.com
Source: slidetodoc.com
The halogen group of elements is the most reactive of the nonmetals. This means it pulls you electrons towards it stronger than any other element. Nov 172:54 pm nonmetal reactivity fluorine has the highest electronegativity and the smallest atomic radius therefore it gains valence electrons the most easily.
 Source: brainly.in
Source: brainly.in
Florine (group 17 most reactive) which element in period 4 is classified as an active metal? Reactivity of metals is based on processes such as the formation of halide compounds with halogens and how easily they displace hydrogen from dilute acids. The halogen group of elements is the most reactive of the nonmetals.
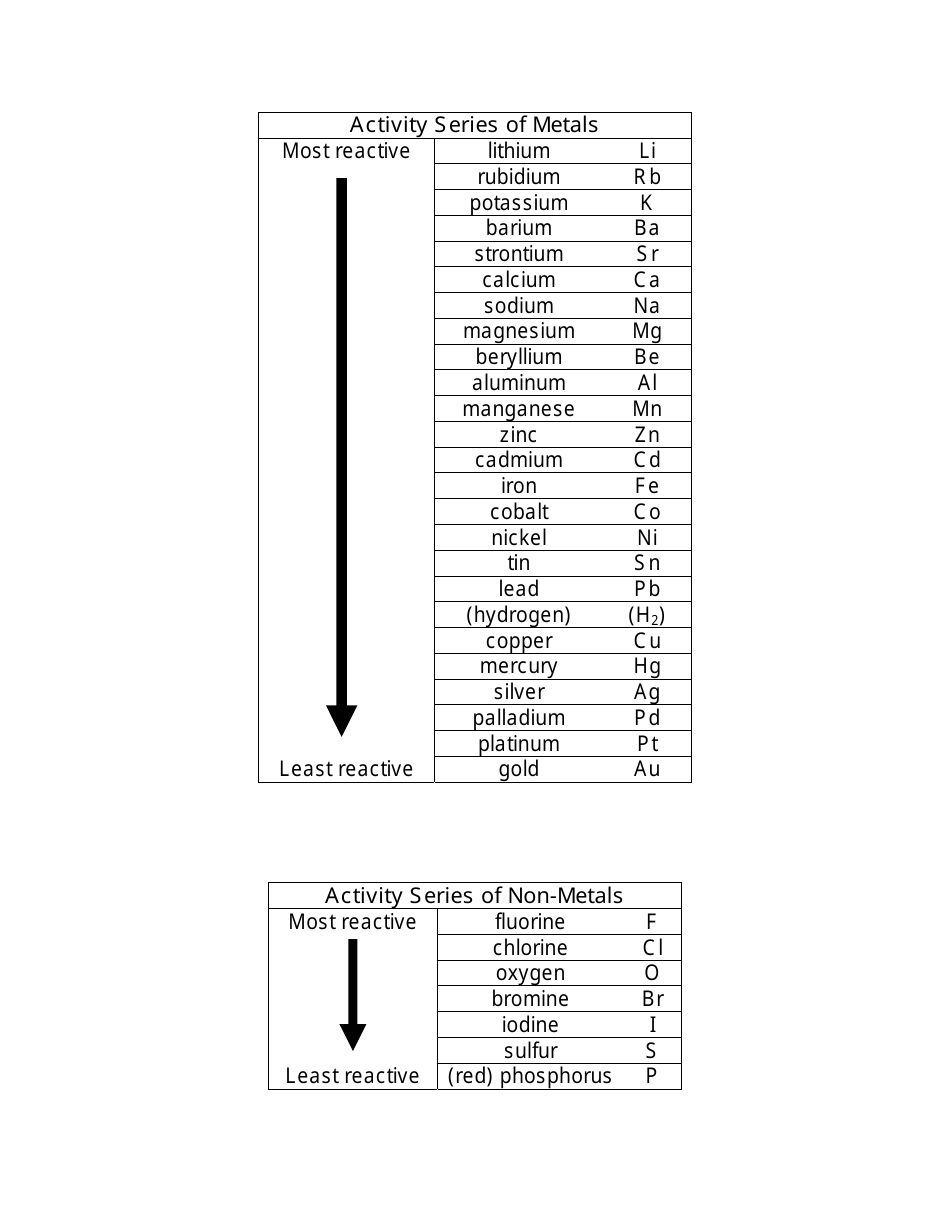 Source: templateroller.com
Source: templateroller.com
Reactivity of metals is based on processes such as the formation of halide compounds with halogens and how easily they displace hydrogen from dilute acids. Hereof, what is the most reactive nonmetal and why? The most reactive nonmetals are those that can most easily gain valence electrons.
 Source: selftution.com
Source: selftution.com
Krypton is a noble gas, so it secretly bonds with every type of element without showing it. The reactive and strongly electronegative nature of the nonmetal halogens represents the epitome of nonmetallic character. Fluorine is the most reactive element in this group.
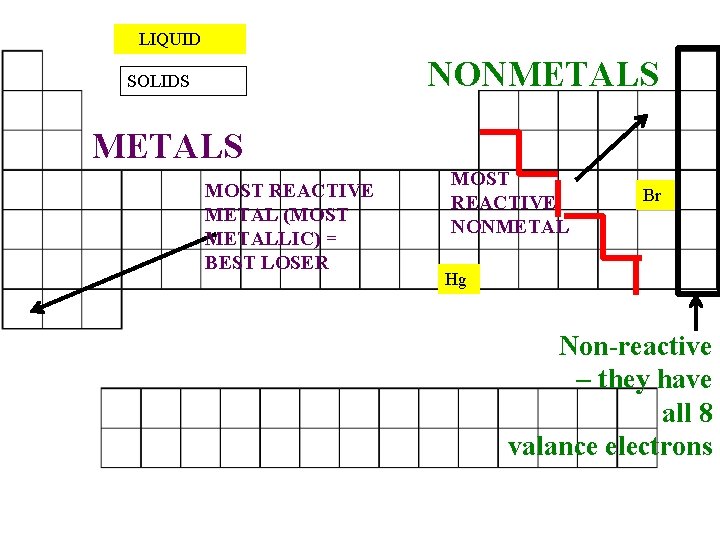 Source: slidetodoc.com
Source: slidetodoc.com
It is also the most reactive group of all chemical elements. Fluorine is the most reactive non metal, since it has the lowest electronegativity of all elements. Looking at metals, theoretically the most reactive metal would be francium.
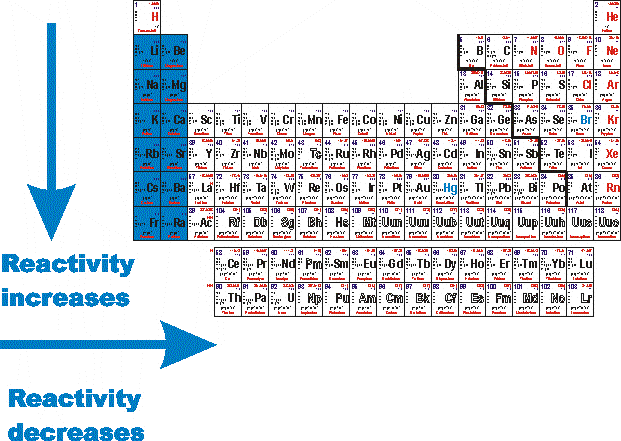 Source: socratic.org
Source: socratic.org
Due to its strong electro negativity & small size, fluorine has a strong tendency to accept electrons from other atoms or ions. Since the noble gases are a special group because of their lack of reactivity, the element fluorine is the most reactive nonmetal. In the nonmetals, reactivity increases as you move up a group, and to the left.
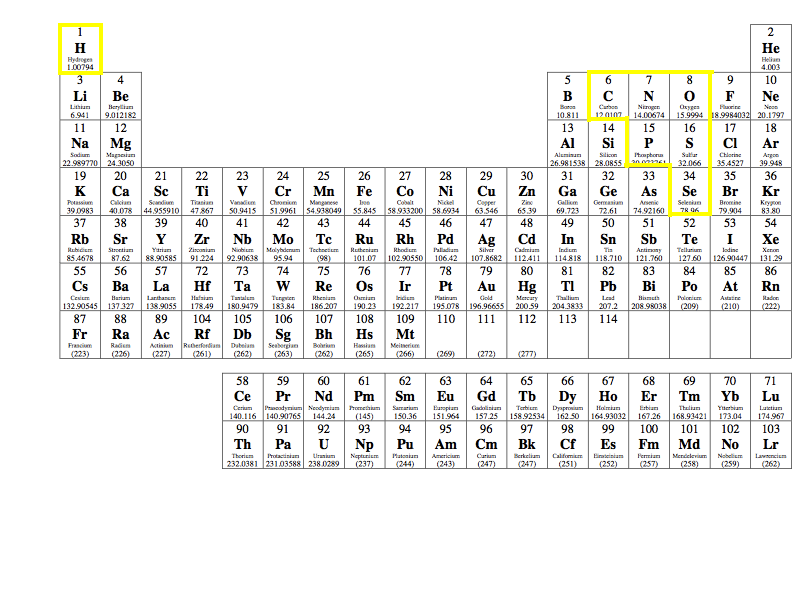 Source: chegg.com
Source: chegg.com
They include lithium, sodium and potassium, which all react vigorously with air and water. Reactivity of metals is based on processes such as the formation of halide compounds with halogens and how easily they displace hydrogen from dilute acids. The most reactive nonmetal is fluorine.
 Source: chegg.com
Source: chegg.com
As its capacity to tempt the shared pair of electron towards itself is more than any type of various other element, fluorine is said to be the a lot of reactive nonsteel in the periodic table. What are the 2 most reactive nonmetals? The most reactive nonmetal is fluorine, f.
 Source: periodictableguide.com
Source: periodictableguide.com
This means it pulls you electrons towards it stronger than any other element. Fluorine is the most reactive nonmetal. This is because they all have one empty space in their valence electron shells.
 Source: breakingatom.com
Source: breakingatom.com
The trend in reactivity in the nonmetals is the opposite of the trend in the metals. This is because they all have one empty space in their valence electron shells. It is not found in nature as a free element.
 Source: youtube.com
Source: youtube.com
The most reactive nonmetal is fluorine, f. This means it pulls you electrons towards it stronger than any other element. Since the noble gases are a special group because of their lack of reactivity, the element fluorine is the most reactive nonmetal.
 Source: socratic.org
Source: socratic.org
Then, which is more reactive metal or nonmetal? Krypton is a noble gas, so it secretly bonds with every type of element without showing it. Florine (group 17 most reactive) which element in period 4 is classified as an active metal?
Also Read :





